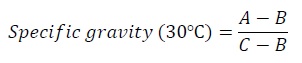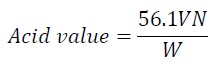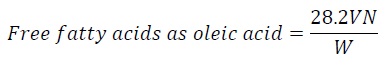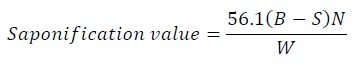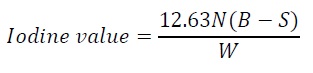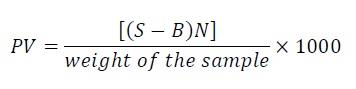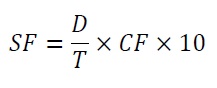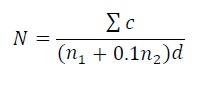Introduction
India enjoys a rich diversity of horticultural crops covering large groups of fruits, vegetables, mushrooms, flowers, plantation and spices. Fruit and vegetable processing is one of the most important and fast-growing sectors of the food processing industry. India ranks second in fruits and vegetables production in the world. Over 90% of India’s exports in fresh fruits and vegetables mainly go to west Asia and East European markets. Most horticultural crops play an important role in human nutrition.
Food which is high in protein content and inexpensive can play an important role to improve malnutrition in developing countries. Most of the currently available functional foods and therapeutic agents are derived either directly or indirectly from naturally occurring sources, especially from plants [1].
With the technological up gradation, there is improvement in processed food product development and quality enhancement. Earlier we had limited categories, now we have innumerable quality enriched food products like jam, jellies, marmalades, squashes, ketchup and sauces in fruit and vegetable sector; processed milk powders and health drinks in dairy sector; bread, biscuits and cakes in bakery sector [2]. Now we are open to a wide range of food products, mainly due to value addition, fortification and functional food development. It is the requirement of food industry and the competitive hotspot to find substitutes of high quality in small cost which can give nutritional enrichment to food products without lowering its taste and nature [3].
Soybeans have been mostly studied apart from oil seeds like melon for fortification and functional food development. Melons are cultivated worldwide for their pleasant flavor and nutritional value. Melon seeds may be eaten as seeds or as fried cake prepared from milled seeds. It is possible that melon seeds could be processed into a product, similar to soybean for protein supply [4]. Melon seeds are rich in potassium and less in sodium; its consumption will help in maintenance of blood pressure and water retention problem in pregnant women. They are also rich source of vitamins and carotenes that adds to the benefit of eye sight, immune system etc. Therefore with all the health benefits melon seeds could be used for production of high quality functional food [1].
The aim of the present study was to characterize and evaluate the phytochemical, fatty acid profile, amino acid profile, antioxidant and antibacterial activities of (C. reticulatus) muskmelon seeds. This study was also conducted to develop and characterize the muskmelon seed fortified biscuits.
Materials and Methods
Raw materials
The Cucumis melo var. reticulates were collected from the Chengalpattu area in Chennai, Tamil Nadu. The seeds were washed and shade dried for two days. The seeds were then hulled manually and stored at room temperature in an air tight container Chemicals were purchased from local supplier for Merck Millipore (Merck Specialities Pvt. Ltd.). Purity: EMPARTAACS grade for analysis. Microbial culture medias and media components were purchased from Hi-media laboratories.
Proximate analyses of Cucumis melo seeds
Proximate analyses for both seed and biscuit were done using the method described by AOAC methods of analysis (AOAC, 1990) [5]. Detailed descriptions of proximate analyses were given in supplementary data.
Extraction and characterization of Cucumis melo seeds polar extract
Extraction: Extraction is the separation of active portions of plant using selective solvents for analysis, characterization and incorporation. Ethanol, acetone, aqueous (water) and chloroform, were the polar solvents used for this extraction. Seeds were extracted by cold process extraction method. Two grams of seeds were weighed and ground by using mortar and pestle with addition of 25 ml to 30 ml of all the above-mentioned solvents, separately. The samples were left in the dark environment for 24 hrs. Then filtration of samples was done by using what man no 1 filter paper. Filtered extract is used for the further analysis.
Qualitative physiochemical analysis: Qualitative physiochemical analyses were done according to the method described by [6]. Detailed descriptions of the methods are given as supplementary data.
Antioxidant analysis: Antioxidant assay on Cucumis melo extracts was estimated for their free-radical scavenging activity by using DPPH (Diphenyl Picryl Hydrazyl) free radicals [7].
Quantitative phytochemical analysis of Cucumis melo seeds
Determination of total phenolic content: The Folin-Ciocalteau reagent method has been used for estimation of phenolic content according to [8].
Determination of Terpenoid content: Hundred grams of seed was soaked in alcohol for 24 hrs and filtered. The filtrate was extracted with petroleum ether. The ether extract was treated as total terpenoid.
Determination of total alkaloid content: The quantification method for alkaloid determination has been used according to [6].
Determination of steroid content: One milliliter of methanolic extract of steroid solution was transferred into 10 ml volumetric flask. Concentrated sulphuric acid (4 N, 2 ml) and iron (III) chloride (0.5% w/v, 2 ml) were added followed by potassium hexacyanoferrate (III) solution (0.5% w/v, 0.5 ml). The mixture was heated in water bath maintained at 70°C ± 2°C for 30 minutes with occasional shaking and diluted to the mark with the distilled water. The absorbance was measured at 780 nm against reagent blank.
Antibacterial activity: Antibacterial activity was determined using Kirby-Bauer disk diffusion method [9]. Test pathogens (Staphylococcus aureus, Bacillus subtilis, Streptococcus pyogens, Pseudomonas aeruginosa and Escherichia coli) were spread on the test plates (Muller Hinton Agar (MHA)) for bacteria using sterile swabs. Sterile wells were made with the help of a sterile cork borer, aseptically. Samples (500 µg, 1000 µg and 1500 µg) were added to the wells at aseptically. Stock solutions of the extracts were prepared using Dimethyl Sulfoxide. The test plates were incubated for 24 hrs. The zone of inhibition (in mm diameter) were read and taken as the activity of the extract against the test organisms.
Extraction and Characterization of Cucumis melo Seed Oil
Muskmelon seed oil was extracted by soxhlet extraction method using n-Hexane as solvent.
Specific gravity: Sample was filled into pycnometer without any air bubbles and side arm was closed. The apparatus was placed in water bath at 30°C for 30 minutes. The cap of side arm was removed and weighed ensuring that the temperature doesn’t fall below 30°C.
Equation 1:
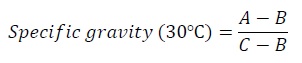
Where,
A - Weight in g of specific gravity bottle with oil at 30°C.
B - Weight in g of specific gravity bottle at 30°C.
C - Weight in g of specific gravity bottle with water at 30°C.
Refractive index: Refractive index instrument calibration was performed by using distilled water which has refractive index of 1.3330 at 20°C and 1.3306 at 40°C. Sample was taken in liquid form, filtered to remove impurities, if present. Sample is added to prism, instrument is adjusted to obtain the most distinct reading. Temperature correction was assayed by following formula.
Equation 2:
R = R1 + K(T1 − T)
Where,
R - Reading of the refractometer reduced to the specified temperature T°C
R1 - Reading at T1°C
K - Constant 0.58 for oils
T1 - Temperature at which the reading R1 is taken and
T - Specified temperature (generally 40°C)
Free fatty acid and acid value: To 5 g of oil 50 ml of fresh neutralized hot ethyl alcohol and 1 ml of phenolphthalein indicator solution were added, boiled for 5 minutes and titrated against standard alkali solution when hot.
Equation 3:
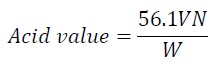
Where,
V - Volume in ml of standard potassium hydroxide or sodium hydroxide used
N - Normality of the potassium hydroxide solution or Sodium hydroxide solution
W - Weight in g of the sample
The acidity is frequently expressed as free fatty acid. Free fatty acid can be calculated by the following formula.
Equation 4:
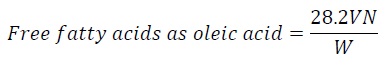
Saponification value: 1.5 g of sample was taken with 25 ml of the alcoholic potassium hydroxide solution and placed in water bath with air condenser. Clarity is achieved in one hour of boiling then excess potassium hydroxide was titrated with 0.5 N hydrochloric acid, using 1 ml of phenolphthalein indicator.
Equation 5:
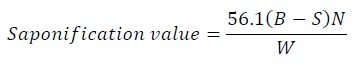
Where,
B - Volume in ml of standard hydrochloric acid required for the blank
S - Volume in ml of standard hydrochloric acid required for the sample
N - Normality of the standard hydrochloric acid and
W - Weight in g of the oil taken for the test
Iodine value: Iodine value was determined by using Wij’s solution. About 2.5 g of oil was taken and 15 ml of carbon tetrachloride, Wij’s solution were added. This was incubated in dark for 30 minutes to 60 minutes and 15 ml 10% KI, 100 ml distilled water were added, titrated with N/10 Na2S2O3. When light yellow color develops starch indicator was added and titrated till white color appears at the bottom.
Equation 6:
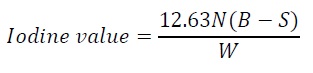
Where,
N - Normality of thiosulphate,
B - Blanktiter value
S - Sample titer value
W - Weight of oil in g.
Unsaponifiable matter: To 5 g of oil 50 ml of alcoholic KOH was added, boiled under reflux air condenser till saponification is complete. The mixture was transferred to separating funnel when warm. Condenser was washed with 10 ml of ethyl alcohol and then with 50 ml water. This was cooled to 20 °C to 25 °C. 50 ml of petroleum ether was added, shaken and allowed for separation of layers. Lower soap layer was transferred to new separating funnel and extraction was performed thrice with 50 ml petroleum ether. The combined ether extract was washed thrice with 25 ml aqueous alcohol followed by 25 ml distilled water. This is transferred to new flask and evaporated to 5 ml. Transfer quantitatively using several portions of ether which were dried previously. After ether evaporation, 2 ml of acetone was added and allowed to dry under air and then dried at 100 °C for 3 min and dissolved in 50 ml of warm ethanol which has been neutralized to a phenolphthalein end point, titrated with 0.02 N NaOH.
Peroxide value: To 5 g sample 30 ml acetic acid chloroform solvent mixture, 0.5 ml saturated potassium iodide solution were added, kept in dark for 1 min with occasional shaking, and 30 ml of water was added. Liberated iodine was titrated with 0.1 N sodium thiosulphate solutions, until yellow color is almost gone. 0.5 ml starch solution was added and titrated until blue color disappears.
Equation 7:
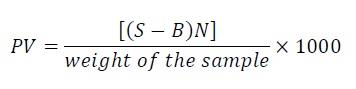
Where,
S – Sample titration value,
B – Blank titration value,
N – Normality of sodium thiosulfate.
Quantitative fatty acid profile estimation: The fatty acid composition in muskmelon seed oil was determined using gas chromatography according to the method described by [10]. Seed oil (100 mg) was weighed into 20 ml test tube and dissolved in 10 ml hexane. Then, 100 µL of 2 N potassium hydroxide in methanol (11.2 g in 100 ml) was added into the test tube, vortexed for 30 s and centrifuged. The clear supernatant (2 ml) was transferred into auto sampler vial and injected into gas chromatography (GCM 5975 C Agilent, USA). The peak areas were computed by integration software and percentages of Fatty Acid Methyl Esters (FAME) were obtained as weight percent by direct internal normalization. National Institute of Standards and Technologies (NIST) USA, Mass Spectra Library was also used as a reference. All analyses were performed in triplicates.
Estimation of Cucumis melo seed amino acid profile: The amino acid analysis was determined using the High Performance Liquid Chromatography (HPLC) specifically the Technichon TSM-1 (techno sequential multisampling) analyzer for amino acid.
Hundred milligram of muskmelon seed sample was weighed into glass ampoule and 7 ml of 6 N HC1 was added and oxygen expelled by passing nitrogen into the ampoule (This is to avoid oxidation of some amino acids during hydrolysis). The glass ampoule was then sealed with Bunsen burner flame put into the oven at 105 °C for 22 hrs. The ampoule was allowed to cool before broken opened at the tip and the content was filtered to remove humins. The filtrate was then evaporated to dryness at 40°C under vacuum in a rotary evaporator. The residue was dissolved with 5 ml of acetate buffer (pH 2.0) and stored in plastic specimen bottles and kept in the freezer. Five microliter to ten microliter of the hydrolyzed sample was loaded into the cartridge of the analyzer (the amount of hydrolyzed sample loaded was dependent on whether the amino acids to be eluted were basic or acidic/neutral. The TSM analyzer is designed to separate and analyze free acidic, neutral and basic amino acids of the hydrolysate. The period of an analysis lasted for 76 minutes. Concentration calculation procedures are given in supplementary data.
Production of Cucumis melo Seed Flour Substituted Biscuits
Biscuit samples were prepared according to the method described by [11]. The formula used for biscuit preparation includes 100 g of wheat flour (Control sample), which was substituted with different proportion (5%, 10%, 15%, 20%, 25%, 30%) of full fat Cucumis melo seeds (Test samples).
Optimization of flour composition for production of Cucumis melo biscuits
The baked samples were evaluated based on their stability and sensory attributes by which the best combination of flour (refined wheat flour + muskmelon seeds) was optimized.
Physical and Sensory Characteristics Analysis of Cucumis melo Biscuits
Sensory analysis: Sensory analysis was done with untrained panel consisting of 25 members. Three samples from two batches of biscuits stored at room temperature were given to the panelists for sensory evaluation. Panelists were asked to give a score for each batch by evaluating all three samples. A nine-point hedonic scale was used for sensory scoring (9 = like extremely, 8 = like very much, 7 = like moderately, 6 = like slightly, 5 = neither like nor dislike, 4 = dislike slightly, 3 = dislike moderately, 2 = dislike very much, 1 = dislike extremely). Characteristics such as color, odor, texture, mouth feel and taste were examined for biscuits and overall acceptability of the product was assessed.
Diameter: To determine the Diameter (D), six biscuits were placed edge to edge. The diameter of six biscuits was measured in mm by using a ruler. The biscuits were rotated at an angle of 90°C for duplicate reading. This was repeated once more and average diameter was reported in millimeters.
Thickness: To determine the Thickness (T), six biscuits were placed on top of one another. The total height was measured in millimeters with the help of ruler. This process was repeated thrice to get an average value and thickness was reported in millimeters.
Spread Factor (SF): SF was determined from the diameter and thickness, with the help of following formula.
Equation 8:
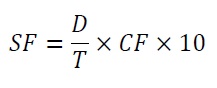
Where,
D – Diameter in mm
T – Thickness in mm
CF - Correction factor at constant atmospheric pressure
Texture analysis: Texture is the property of food which is associated with the sense of feel or touch experienced by fingers or mouth. Texture analysis of muskmelon seed based biscuits was done by using TA-XT Texture analyzer (Stable micro systems). Mini 3 Point Bed Rig probe was used for the analysis. The hardness and fracturability of the biscuits were determined.
Storage studies of Cucumis melo biscuits: Biscuits were divided into 50 g portions, packed in Bi-axially Oriented Polypropylene (BOPP) film bags, sealed and stored in air tight container at room temperature for a period of 45 days. Samples were drawn periodically and evaluated for microbial analysis, protein content, moisture uptake and peroxide value at every 15 days interval. Moisture and peroxide values were estimated as explained in previous sections 2.1 (Supplementary data) and 2.4.7, respectively.
Microbial analysis: Sample was prepared in 1:10 dilution by aseptically homogenizing 25 g of biscuit in 250 ml of the sterilized distilled water. Pour plate technique was used for bacteria and spread plate for yeast. Nutrient agar and potato dextrose agar plates were used for bacterial (37°C for 48 hrs) and yeast (25°C for 3 days to 5 days) enumerations, respectively. Colonies were calculated using the following formula.
Equation 9:
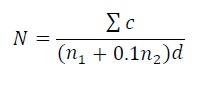
Where,
N - Plate Count in sample
Σc- The total number of colonies on the plates (including the plates within the range of proper plate count)
n1 - The number of colonies on the plates of the first proper dilution degree
n2 - The number of colonies on the plates of the second proper dilution degree
d - Dilution Factor (the first dilution degree)
Statistical analysis: All the reported analyses were performed in triplicates and the values were given in terms of mean and standard deviation. Analysis of variance test and Duncan's multiple range tests was done for mean values of analyses to evaluate the significant differences between samples. Microsoft Excel Data Analysis Centre (2010) and IBM SPSS software (version 22) were used for statistical analysis.
Results and Discussions
Proximate analysis of Cucumis melo seed powder
The ground C. reticulatus seeds were found to contain 5.50% ± 0.05% Moisture, 25% ± 0.03% Protein, 4.60% ± 0.12% Ash, 34.05% ± 0.06% Fat/Oil, 1.75% ± 0.02% Crude fiber, 16.31% ± 0.52% Carbohydrate, respectively. These results indicate that C. reticulatus seeds are rich in fat, protein and carbohydrate. Oil content of C. reticulatus seeds was found to exceed, or comparable to, that of some common edible oils such as cotton seed (22% to 27%), safflower (30% to 35%), soybean (18% to 22%) and olive (12% to 50%) [12]. Therefore, the C. reticulatus seeds can be considered as a potential source of vegetable oil for domestic and industrial purposes.
Extraction, screening and characterization of Cucumis melo seed polar extracts
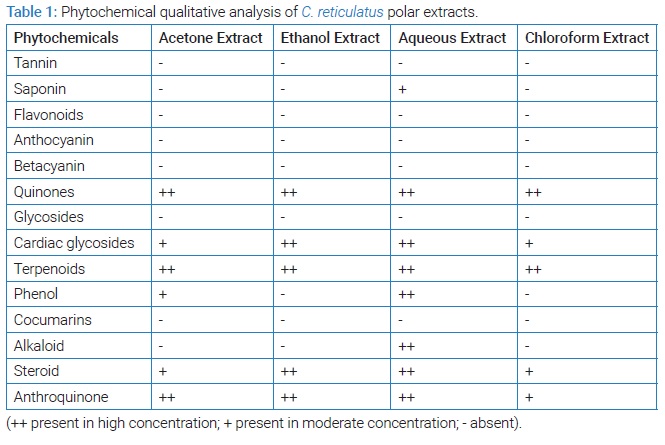
Qualitative phytochemical analysis of Cucumis melo seed polar extracts: Results of the qualitative phytochemical analysis of C. reticulatus seeds were tabulated in (Table 1). The preliminary phytochemical analysis revealed the presence of various phytochemicals such as Saponin, Quinones, Cardiac glycosides, Terpenoids, Phenol, Alkaloid, Steroid, and Anthroquinone. The aqueous extract was examined to be rich in steroid content and only moderate amount of steroid were present in acetone and chloroform. Generally steroids are less polar compounds and thus they are readily soluble in non-polar solvents and sparingly soluble in polar solvents. In our preliminary qualitative analysis, steroids were examined to be rich in aqueous extract; this anomaly may be attributed to presence of cucurbitacin molecules in melon seeds. The cucurbitacin and sterol molecules can only be separated and estimated through advance HPLC analysis [13]. Higher concentrations of phenols, terpenoids, quinones and cardiac glycosides were present in aqueous extract of C. reticulatus. Tannin, flavonoid, glycoside and coumarin were absent in all the extracts. Presence of alkaloids was found to be high in aqueous extract for C. reticulatus.
Antioxidant analysis of Cucumis melo seed polar extracts: The DPPH method was selected for measuring the primary antioxidant activity of the seed extracts because it is one of the most effective methods for evaluating the concentration of radical-scavenging material activity by a chain-breaking mechanism [14]. The decolorisation of the purple reaction solution is stoichiometric with respect to number of electrons captured [15,16]. Aqueous extract of C. reticulatus seeds showed maximum free radical-scavengin activity (64.29% ± 8.32% inhibition of DPPH) followed by ethanol extract (55.71% ± 6.24%), chloroform extract (47.14% ± 5.37%) and acetone extract (37.21% ± 5.11%).
Qualitative phytochemical and antioxidant analysis of C. reticulatus seeds revealed that the aqueous extract of the seeds possess high amount of antioxidants and phytochemicals. Thus aqueous extract of C. reticulatus seeds was screened for further quantitative analyses.
Characterization of Cucumis melo seed aqueous extract
Quantitative phytochemical analysis of Cucumis melo seed aqueous extract: Aqueous extract of C. reticulatus seeds were found to have high amount of terpenoids (110 mg/g), followed by steroids (19 mg/g), alkaloids (13.96%) and total phenols (9.2 mg GAE/g).
Gallic acid was used as standard for total phenol estimation. Quantitative estimation has proved the chemical basis for wide use of this seed as therapeutic agent for treating various ailments.
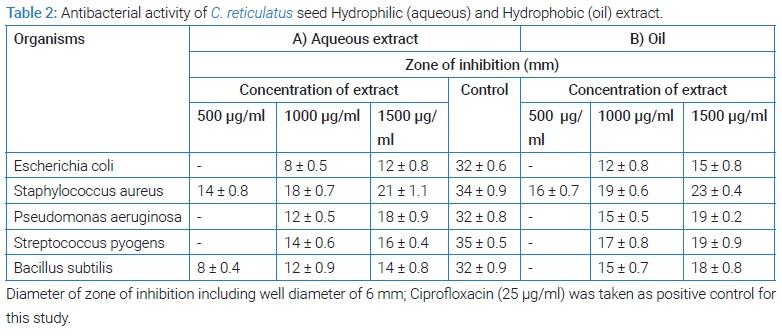
Antibacterial analysis of Cucumis melo seed aqueous extract: (Table 2A) shows the results of antibacterial activity of aqueous extract of C. reticulatuss seeds. The aqueous extract of seeds have shown antibacterial activity against gram positive microorganisms such as Staphylococcus aureus, Bacillus subtilis, Streptococcus pyogens at different concentrations as compare to standard aqueous extract. The higher concentration of extract (1500 µg/ml) inhibited the growth of Staphylococcus aureus by 21 mm, the same concentration of extract showed a growth inhibitory zone measuring 12 mm towards Escherichia coli. The aqueous extract of all concentrations significantly inhibited growth of Staphylococcus aureus. Staphylococcus aureus was found to be the most sensitive and Escherichia coli the least sensitive organism for aqueous extract of C. reticulatus seeds.
Extraction and characterization of Cucumis melo seed oil (non polar) content
Oil was extracted from C. reticulatus seeds using soxhlet extraction method with n-Hexane as solvent. The yield of extracted oil from seed kernel of C. reticulatus was found to be 34.05% ± 2.5%. This extraction yield exceeds the range (22.7% to 30.4%) of previous extraction report [17].
Physical and chemical characteristics of Cucumis melo seed oil: Physical properties of lipids derive directly from their chemical structure and functional groups and greatly influence the functions of lipids in foods and the methods required for their manipulation and processing. They can also be used to assess the purity or quality of lipid material in reference to know standards or preferred characteristics [18].
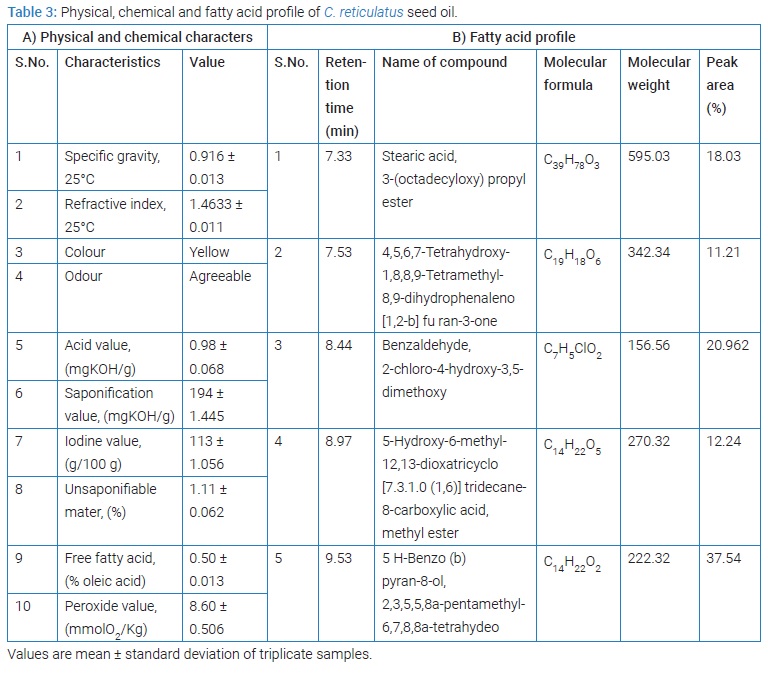
Physiochemical characteristics of C. reticulatus seed oil has been tabulated in (Table 3A). C. reticulatus seed oil was liquid at room temperature (25°C) and has yellow color with agreeable flavors. Refractive index of C. reticulatus seed oil was found to be 1.4633 ± 0.011. This value is in the range reported for the pumpkin seed oil (1.466–1.474) and it is lower than that reported for sunflower and olive oils [18]. High saponification value shows that fatty acids present in oil have a high number of carbon atoms. The free fatty acid as linoleic acid in C. reticulatus seed oil was found to be 0.50% ± 0.13% and it is agreement with previous report on pumpkin seed oil [19]. The low peroxide value of C. reticulatus seed oil indicates low level of oxidative rancidity of oil and also suggests the presence or high levels of antioxidants.
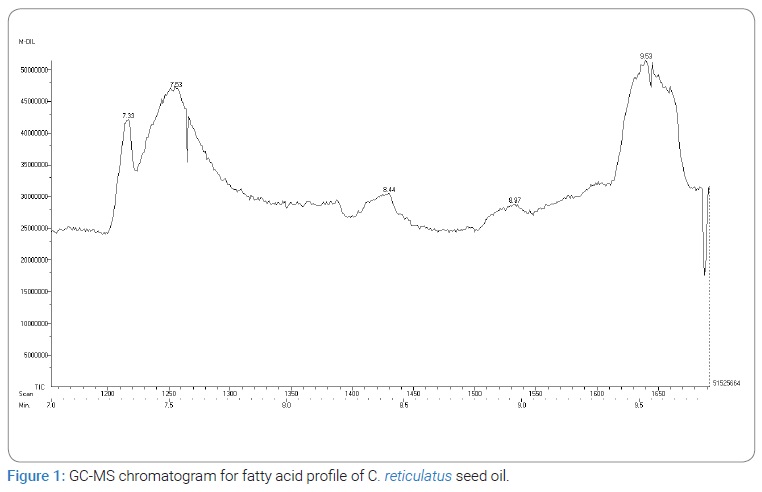
Quantitative fatty acid profile analysis of Cucumis melo seed oil: GC-MS spectral chromatogram of C. reticulatus seed oil depicted in (Figure 1), confirmed the presence of linoleic acid as major constituent of C. reticulatus seed oil. Based on the peak area (%), Retention Time (RT), Molecular Weight (MW) and Molecular Formula (MF) five major constituents of the oils were recognized using National Institute of Standards and Technologies (NIST) USA, Mass Spectra Library. The recognized components were tabulated in (Table 3B).The results suggest that melon seeds especially kernels contain high amount of oil. The seed oil contains linoleic acid as major fatty acid. The seeds could be extracted for oil and further more used for edible purpose. The results obtained was found to be in agreement with the fatty acid content of citrullus colocynthis L seeds, which was reported to have 62.2% of linoleic acid as major fatty acid [20]. Similar results were also observed in pumpkin seed oil which showed 42% of linoleic acid as major fatty acid followed by oleic acid [21].
Antibacterial analysis of Cucumis melo seed oil: Results of the antibacterial analysis of C. reticulatus seed oil are tabulated in (Table 2B). Oil extracted from muskmelon seeds showed comparatively higher antimicrobial effect than their aqueous extracts. Minimum inhibitory concentration of muskmelon oil for examined panel of bacteria was found to be 1000 µg/ml. It was found that both the aqueous extract and oil of musk melon seeds have high antibacterial activity against Staphylococcus aureus. But C. reticulatus seed oil was found to have comparatively high antibacterial activity against both Escherichia coli and Bacillus subtilis, than the aqueous extract.
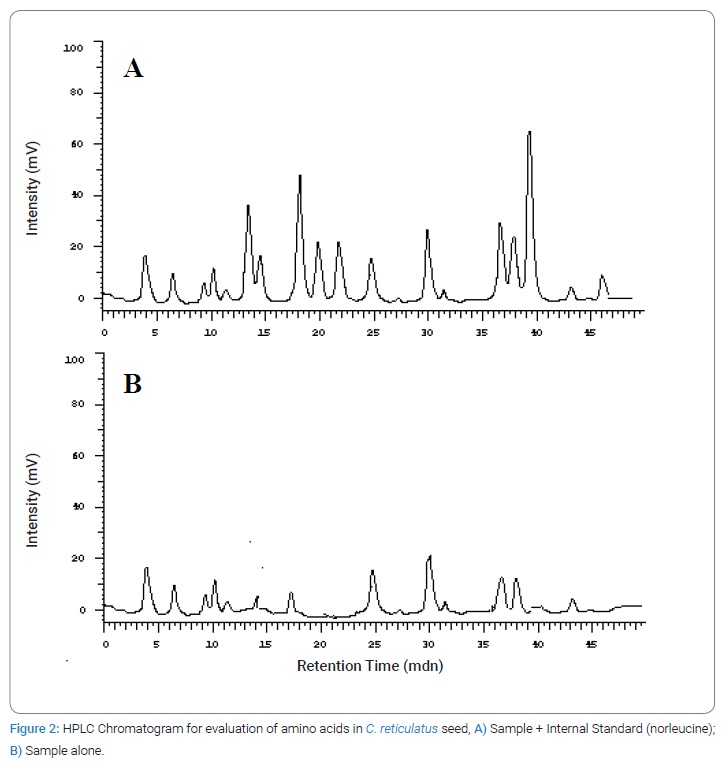
Amino acid profile of Cucumis melo seeds: The amino acid profile of C. reticulatus seed was examined, via HPLC analysis, using nor leucine as internal standard. The standard and sample chromatograms are depicted in (Figure 2A and 2B). The quantitative estimation of amino acids present in C. reticulatus seed was done using difference in peak area of amino acids and known internal standard concentration [22]. The seeds of C. reticulatus were found to be rich in amino acids such as glutamic acid (RT 6.46 min, 367 mg/100 g protein), aspartic acid (RT 4.04 min, 239 mg/100 g protein) and threonine (RT 14.91 min, 155 mg/100 g protein) present in major quantities. It is well known that aspartic acid and glutamic acid contribute to the pleasant umami taste or savory enhancement in foods [23]. Glutamic acid is the most important flavor enhancing amino acid. Other minor amino acid includes Asparagine (RT 9.26 min, 73.1 mg/100 g protein), Phenyl alanine (RT 37.93 min, 55.1 mg/100 g protein), Tyrosine (RT 24.86 min, 54.6 mg/100 g protein), Glycine (RT 13.47 min, 52.4 mg/100 g protein), Iso-leucine (RT 36.62 min, 51.3 mg/100 g protein), Serine (RT 10.24 min, 44.1 mg/100 g protein), Methionine (RT 31.42 min, 38.4 mg/100 g protein), Valine (RT 29.91 min, 35.2 mg/100 g protein), Leucine (RT 39.34 min, 32.4 mg/100 g protein), Gultamine (RT 11.69 min, 30 mg/100 g protein), Cystine (RT 21.78 min, 25.1 mg/100 g protein), Alanine (RT 19.56 min, 24.5 mg/100 g protein), Histidine (RT 27.25 min, 19.4 mg/100 g protein), Arginine (RT 18.09 min, 3.5 mg/100 g protein) and Lysine (RT 43.39 min, 3.5 mg/100 g protein).
Production, Optimization and Characterization of Cucumis melo Seed Biscuits
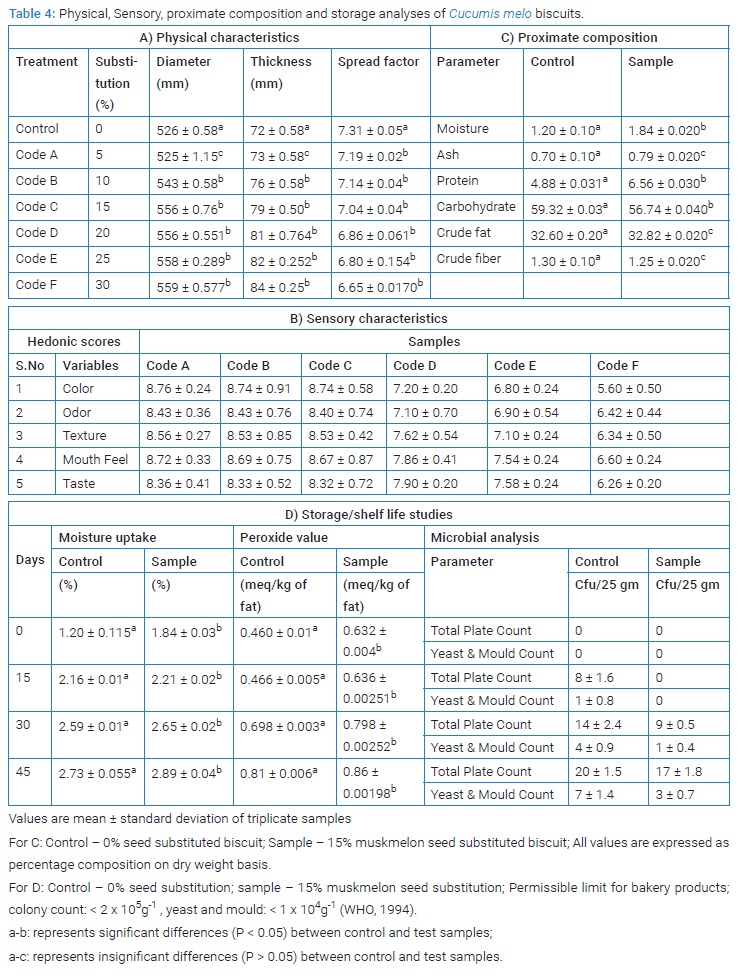
Physical characteristics of Cucumis melo seed biscuits: Physical characteristics of biscuits are important for both consumers as well as manufactures point of view. The effect of substituted wheat flour with 5%, 10%, 15%, 20%, 25% and 30% full fat ground C. reticulatus seeds in physical characteristics of biscuits was studied and the data are presented in (Table 4A).The results showed that all full fat ground C. reticulatus seeds treatment caused significant (P < 0.05) increase in biscuits width as compared with 526 mm ± 0.58 mm for control and 5% of full fat seeds (525 mm ± 1.15 mm) respectively. It is clear that biscuits substituted wheat flour with 30% ground seeds had maximum width (559 mm ± 0.5774 mm) followed by 25% ground seeds (558 mm ± 0.289 mm).
There was no significant (P > 0.05) difference in width of 15% and 20% seed substituted biscuits. While minimum width (525 mm ± 1.15 mm) was observed in biscuits substituted wheat flour with 5% ground C. reticulatus seeds.
Sensory analysis of Cucumis melo seed biscuits: Sensory hedonic scale scores for Cucumis melo seed biscuits are given in (Table 4B). Code A, B & C, samples with 5%, 10%, 15% Cucumis melo seed flour substituted biscuits was found to have higher sensory scores for all five examined variables (Color, Odor, Texture, Mouth feel and Taste). As the concentration of Cucumis melo seed flour substitution increases the sensory scores for the biscuits were found to be decreased, which suggest that the addition of excess Cucumis melo seed flour depletes the sensory characteristics of biscuits, which in-turn reduces overall acceptability of consumers. But drastic decrease in sensory scores of all five variables was noted between Code C to Code D. 15% Cucumis melo seed flour substitution was found to be the limit for retention of sensory properties of biscuits, thus Code C samples were taken for further storage studies.
Texture analysis of Cucumis melo seed biscuits: The hardness (force), fracturability (distance) of the biscuits was analyzed using the texture analyzer. The hardness of both control and muskmelon biscuits were found to be 1546.391 g ± 0.399 g and 1256.29 g ± 0.6525 g, which is in comparable range with breaking strength of wheat-plantain biscuits (1.9 kgs) and wheat biscuits (1.10 kgs). It was however lower than, breaking strength (3.45 kgs) of complete plantain biscuits (without wheat or maida) [24]. Fracturability of the biscuits was found to be 10.687 mm ± 0.02 mm and 9.574 mm ± 0.304 mm respectively, for muskmelon seed biscuits and control samples. The low biting/breaking strength along with high hardness gives an added advantage to the biscuits as the shape can be maintained during the storage and transportation, without any difficulty in biting and chewing.
Proximate analysis of Cucumis melo seed biscuits: The results tabulated in (Table 4C), shows that the moisture content of biscuit samples was 1.2% ± 0.1% for control and 1.84% ± 0.02% for muskmelon seed biscuits. The increase in moisture content of biscuits substituted with full fat ground Cucumis melo seeds may be due to its ability to absorb water. This observation is in agreement with other research reports [25]. Also biscuits substituted with full fat ground C. reticulatus seeds had higher content of protein than that of control samples. The carbohydrate content was found to be reduced in the muskmelon seed biscuits than the control sample.
Storage studies of Cucumis melo seed biscuits: The storage study results of Cucumis melo seed biscuits are tabulated in (Table 4D).
The initial moisture content of C. reticulatus seed biscuits was 1.84 ± 0.036, increased significantly (P < 0.05) up to 2.89 ± 0.040 by the end of ambient storage of 45 days. The moisture uptake might be attributed by diffusion of gases.The peroxide value was found to increase with time. The initial peroxide value was 0.46 meq/kg ± 0.01 meq/kg fat. The increase in peroxide value percentage was less in muskmelon seed biscuit. This may be due to the antioxidant capacity of muskmelon seeds. But initial peroxide value of muskmelon seed biscuit was found to be significantly more than the control biscuit, which may be due to high amount of free fat content in muskmelon seeds. This results are comparable to the barnyard biscuit- wheat biscuit, where the peroxide liberation was initially 0.28 meq/kg fat which after second month showed drastic increase percentage of 20 [26].
Microbial analysis for storage study of biscuits showed that the biscuits made with substitution of full fat ground muskmelon seeds had lower microbial loads than the control biscuits. The least bacterial population of 9 Cfu/25g ± 0.5 Cfu/25g was observed in muskmelon seed biscuits at 30 days of storage. The highest bacterial population was observed in muskmelon seed biscuit (17 Cfu/25g ± 1.8 Cfu/25g) at 45 days of storage. The maximum yeast and mould growth (7 Cfu/25g ± 1.4 Cfu/25g) was found at 45 days after storage. The relatively presence of bacteria and moulds might be due to the processing, which muskmelon seeds are subjected to. However, high temperature of baking is expected to destroy all the microorganisms present. Microbial load of biscuits was in acceptable limit for a period of 45 days from manufacture. The bacterial count of all biscuit samples was lower than acceptable limit of 1 x 105 Cfu/g of sample. The value obtained from the present study was found to be lower than that of potato flour incorporated biscuit samples which had 4.5 x 103 Cfu/g, and 6.5 x 103 Cfu/g, at 30 days and 60 days of storage, respectively [27].