Neurodevelopmental Status Evaluation following up Children Born with Term Intrapartum Asphyxia from 2 to 5 Years of Age
Zarrin Keihani-Doust;
* Maryam Saeedi;
Rezvan Ashkanipour;
Zeinab Kavyani;
Mamak Shariat;
Fatemeh Tehrani;
-
Zarrin Keihani-Doust: Department of Pediatrics, Imam Hospital, Tehran University of Medical Sciences, Tehran, Iran.
-
* Maryam Saeedi: Children's Medical Center, Tehran University of Medical Sciences, Tehran, Iran.
-
Rezvan Ashkanipour: Tehran University of Medical Sciences, Tehran, Iran.
-
Zeinab Kavyani: Breast Feeding Research Center, Tehran University of Medical Sciences, Tehran, Iran.
-
Mamak Shariat: Maternal, Fetal and Neonatal Research Center, Tehran University of Medical Sciences, Tehran, Iran.
-
Fatemeh Tehrani: Department of Pediatrics, Imam Hospital, Tehran University of Medical Sciences, Tehran, Iran.
-
Mar 18, 2022 |
-
Volume: 1 |
-
Issue: 1 |
-
Views: 1217 |
-
Downloads: 1140 |
Abstract
Background: Hypoxic-ischemic encephalopathy is a significant cause of permanent damage to brain cells and neurodevelopmental problems in infants.
Methods: In this prospective cohort study, 27 infants born between 2007 and 2011 were studied. In a previous survey conducted in 2012, the first two years of the lives of these infants were investigated for neurodevelopmental status. During the previous study, 3 cases were lost and 27 cases were enrolled in the present study. To evaluate neurodevelopment, Ages & Stages Questionnaires (ASQ) was completed for these children.
Results: Three of 27 (11.1%) neonates born with asphyxia died, all of whom had severe encephalopathy and multiorgan involvement. Among the remaining 24 patients, two cases (8.3%) scored lower than the specified cut-off in some areas.
Conclusion: In our study, scores of two patients (8.3%) of those who remained were lower than the determined cutoff. Long-term neurodevelopmental outcomes should be considered regularly to discover permanent problems.
Introduction
Cerebral hypoxemia ischemia leads to hypoxic-ischemic encephalopathy with clinical symptoms which are significant cause of permanent damage of brain cells and neurologic disability in newborns. Each year, nearly 4 million infants are born with asphyxia worldwide, a million of them die, and one million of those who survive to suffer from serious complications such as cerebral palsy, mental retardation, and seizures [1]. The combinations of basic criteria which form hypoxic-ischemic encephalopathy are fetal distress in the last hours before birth, respiratory depression at birth, need for resuscitation, severe metabolic acidosis, changes in clinical signs of infants (alertness, muscle tone, body posture, tendon reflexes, myoclonus, Moro reflex, corneal condition, seizures), multiple organ failure, abnormal Electroencephalogram (EEG) findings and lack of other causes of neonatal encephalopathy [2–4]. Hypoxic-ischemic encephalopathy based on clinical symptoms is ranked into three stages: Stage 1 with a good prognosis, stage 2 with a varied prognosis, and stage 3 - or prolonged stage 2 of more than 7 days or lack of EEG findings that revert to normal - with a prognosis of death and severe injuries. Therefore, the expected outcome in newborns with asphyxia is in a spectrum from normal to neurodevelopmental disabilities and death [5,6].
Currently, there is no global consensus about a gold standard for the diagnosis of asphyxia [7]. However, based on previous studies, three sets of definitions are provided for intrapartum hypoxia. According to the American Academy of Pediatrics and the American College of Obstetrics and Gynecology, profound acidosis (cord blood pH < 7.0), Apgar score < 3 at 5th minute of birth and beyond, neonatal encephalopathy, and multiorgan system failure indicate intrapartum hypoxia [8,9]. According to the International Cerebral Palsy Task Force in 1999, intrapartum hypoxia is defined by infant blood pH < 7 in the first blood sample and a base deficit > 12 mmol/L (metabolic acidosis), moderate to severe encephalopathy, and cerebral palsy [9]. According to the American College of Obstetrics and Gynecology in 2003, metabolic acidosis (pH < 7 and a base deficit > 12 mmol/L) and moderate to severe encephalopathy are essential criteria for intrapartum hypoxia [10]. Previous studies have shown that pH and 5th minute Apgar score has limited value in predicting the neurodevelopmental status of hypoxic-ischemic infants. The severity of encephalopathy due to asphyxia may be the best determining factor [2].
In recent years, the uncomplicated survival of very low birth weight infants in most hospitals has significantly increased. However, the mortality and morbidity of term infants born with asphyxia have not decreased greatly and asphyxia is one of the most important risk factors for developmental complications [11]. The time and type of childhood follow-up after hypoxic-ischemic encephalopathy can be especially helpful in identifying the following disorders: severe sensory or motor impairment (first year), growth deficit (second year), impaired fine and gross motor function (2 years to 4 years of age), cognitive impairment (4 years to 7 years of age) and learning deficit (7 years to 9 years of age) [6].
Only a few studies are available on the accurate assessment of hypoxic-ischemic encephalopathy and the long-term consequences so, given that there is no specific treatment for CNS damage, it is important to prevent encephalopathy followed by asphyxia [12]. In a study from 2007 to 2011 in Tehran, the capital city of Iran, 27 neonates of them neonates born with asphyxia were followed up until 2 years of age and evaluated in terms of neurodevelopmental status [13]. The present study is a continuation of the 2007 to 2011 study in which the neurodevelopmental status from 2 years to 5 years of age is evaluated through completion of the ASQ.
Methods
In this prospective cohort study, 27 infants born with asphyxia were investigated in a university-based pediatric hospital from 2007 to 2011 in Tehran, the capital city of Iran. Undertaking that study in 2012 [13], the neurodevelopmental outcomes of the first two years of life were evaluated. Three of the neonates had died during the first two years of life and three infants were lost during the present study - two of the families were out of reach and the parents of one child were not willing to cooperate. Finally, 24 neonates were enrolled for this step of the follow-up.
Hypoxia has been defined using 3 major and 5 minor criteria. Cord blood pH < 7.2, infant blood pH < 7.2 and a base deficit > 12 mmol/L in the first-hour blood sample were the major criteria. The minor criteria consisted of Apgar score < 5 at the 5th minute or beyond, the need for immediate neonatal resuscitation, the presence of meconium-stained liquor, abnormal EEG, and acute changes in imaging studies. The presence of one major criterion and at least two minor criteria were diagnosed as intrapartum hypoxia. Neonates with gestational age less than 37 weeks, metabolic disorders, and congenital abnormalities were excluded.
The ASQ was selected for assessing neurodevelopmental status following asphyxia. This questionnaire examines 5 different areas of neurodevelopmental conditions: communication, gross motor (including the head lag, walking, sitting, standing, and running), fine motor (including gripping, grasping, playing with cubes, and performing fine movements with hands and feet), problem-solving (as mental health and ability to select the proper ways for doing things) and personal-social area (ability to communicate, understand a game and ability to perform activities correctly). For each question, a score of 10 was allocated to the child's ability, a score of zero to his/her inability and a score of 5 to relative ability to perform activities. The questionnaires were then scored based on the available cutoff. The questionnaires were completed by someone who regularly spent time with the child [14]. This questionnaire is performed at 2 months intervals for children between 4 months to 24 months of age, at 3 months intervals for children from 25 months to 36 months of age, and at 6 month intervals for children from 37 months to 60 months of age; therefore, 8 questionnaires were considered for each child at 27 months, 30 months, 33 months, 36 months, 42 months, 48 months, 54 months and 60 months.
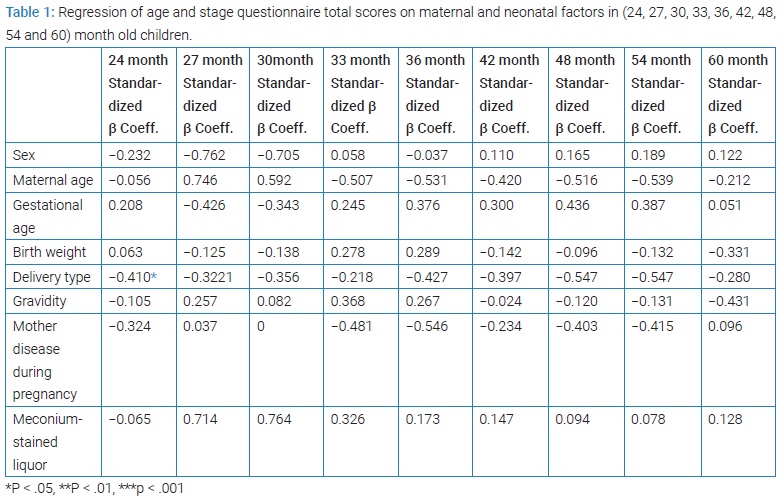
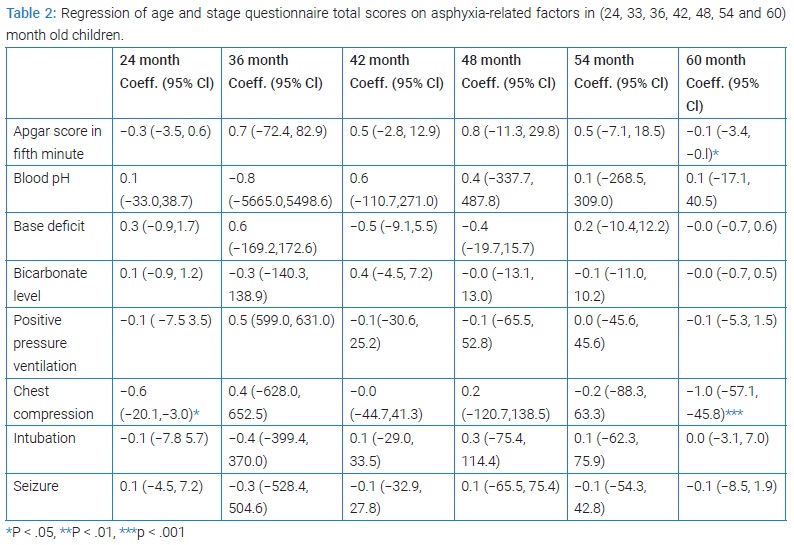
The effects of several maternal and neonatal variables on the ASQ score were assessed. The maternal variables included age at pregnancy, gestational age, gravidity, delivery type (cesarean section versus normal vaginal delivery), and the existence of any chronic diseases during pregnancy (e.g., diabetes mellitus, chronic hypertension, infectious diseases with systemic manifestations, chronic renal disease, eclampsia and pre-eclampsia, hypothyroidism, asthma, hemoglobinopathies, platelet disorders, immunologic disorders, and other chronic and systemic disorders). The neonatal variables included birth weight, Apgar score at the fifth minute, meconium-stained liquor, blood pH, base deficit, bicarbonate level, chest compression, Positive Pressure Ventilation (PPV), intubation, and occurrence of seizure.
At the end of each questionnaire, a series of general open-ended questions without scoring was asked – concerning hearing ability, vision, position of children compared with peers and parents' concerns about their children – and evaluated.
Statistical analysis
Scores were calculated by converting answers to numerical equivalents; 10 points for 'yes', 5 for 'sometimes', and 0 for 'not yet' answers. Data were analyzed using STATA 10 software (Stata Corporation, College Station, TX). To compare the means of the subcategories of patients, a Student's t-test was used. The means of numerical variables were compared by the Kruskal-Wallis test and categorical data were analyzed using chi-square and Fisher exact tests. Linear regression was used to univariate comparisons of maternal and neonatal variables with the ASQ scores. A p-value < .05 was considered to be significant.
Results
Thirty children born with asphyxia at Vali-Asr Hospital in the Imam Khomeini Medical Complex between October 2007 and October 2011 were enrolled for an initial survey in which 3 cases died (2 on the first day and one on the 19th day of life) and neurodevelopment outcomes were studied for the first two years of life for the remaining 27 cases. In the present stage of the study, 3 patients have been excluded: 2 patients due to lack of access and 1 due to lack of cooperation. The remaining 24 children were evaluated for neurodevelopmental status from 2 years to 5 years of age. These children were 18 children (75%) males and 7 children (25%) females. Of these, 15 children (62.5%) were delivered by Cesarean Section (SC) and 9 children (37.5%) by Normal Vaginal Delivery (NVD). 55.6% of infants were the first child of the family and were born at 37 weeks of gestational age (29.6%). Newborns that were resuscitated after delivery and received PPV comprised 81.5% of these infants (n = 22). For 22.2% (n = 6), chest compression was administered and for about 22.2% (n = 6), endotracheal intubation was performed. Six cases (22.2%) had seizures in the first three days of life. EEG was done for 5 of these neonates and 3% of these 5 (60%) had ictal discharges in their EEG. Ultrasonography was performed on 21 children, 5 (18.5%) of whom had abnormal findings. CT brain scans were carried out on 7 children 3 (42.8%) of which were abnormal. The abnormalities were Intraventricular Hemorrhage (IVH), Periventricular Leukomalacia (PVL).
Among the 27 infants, the Birth weight of the newborns was mostly between 3 kg to 4 kg (59.3%). Most of the infants had 5th minute Apgar scores above 5 (92.6%). Most had blood pH at the first hour of birth between 7 and 7.2 (14 participants). Thirteen cases (48.2%) had base deficit > -10, and 6 patients (22.2%) had base deficit < -14. The bicarbonate level for 66.7% of the children was < 18. < 7 days of hospitalization was documented for 59.2% of the children.
Six children, born in 2011, were 24-month-old at the beginning of this survey and thus completed the questionnaires for 24 months to 42 months of age. All six children in all 5 studied areas had mean ASQ scores higher than the cutoff point. For one child questionnaires were completed at 36 months, 42 months, 48 months, and 54 months, one child 36 months to 60 months, one child 54 months and 60 months, one child 48 months, 54 months, and 60 months and in all 5 areas mean scores were higher than the determined cutoff (the 24 month questionnaire was completed in the previous study). For 7 children 42 months to 60 month forms were completed (the 24 month questionnaire was completed in the previous study) one of which showed mean scores lower than the cutoff in the 24th month with low scores in the fields of communication and gross motor development. At 42 months to 60 months this child in all five studied areas had less than the cutoff scores. The 33 months to 60 month questionnaires were completed for 2 children, one of which had a mean score lower than the cutoff for all 5 areas. Five patients were at the 60th month of age at the time of the survey, so only the 60 month questionnaire was used and the scores of all of these 5 children were higher than the cutoff in all 5 domains (they had completed the 24 month questionnaire in the previous study).
Some variables (including sex, age, delivery type, birth weight, gestational age, gravidity, maternal disorders during pregnancy, and meconium staining) associated with asphyxia in the process of infant neurodevelopment were regressed to investigate the confounding effects. Each of these variables was imported into the model separately and excluded from the final analysis if it was not significantly related to the neurodevelopment process model.
ASQ for 24-month-old children
At 24 months of age, type of delivery - as a risk factor - was significantly associated with neurodevelopmental status (P = 0.042) (Table 1). In linear regression at 24 months, resuscitation by heart massage showed a significant association with neurodevelopmental condition (P = 0.011) while other parameters including 5th minute Apgar score, blood pH, base deficit and bicarbonate level, neonatal resuscitation (PPV, chest compressions, endotracheal intubation) and seizures were not significantly related to the neurodevelopmental status (Table 2).
Concerning the open-ended questions, all 24-month-old children, according to their parents, had good hearing and vision and spoke understandably. Two (9.1%) believed that their child did not speak like their peers and one of them had an ASQ score lower than the cutoff in the area of communication. One parent (4.5%) said that their child did not walk like other children and this child had lower ASQ scores in the gross motor category. Two families had previous histories of the problem with children in their family. Six people were concerned about their children (stuttering, hypothyroidism, motor retardation, underweight, hyperactivity, and allergies).
ASQ for 27-month-old children
In the 27th month, none of the variables (sex, age, delivery type, birth weight, gestational age, gravity, disorders of the mother during pregnancy, and meconium staining) were associated significantly with the scores (Table 1).
In open-ended questions, all 27-month-old children were considered to have good hearing and vision, speak understandably, and walk like peers. One family had a history of trouble in a previous birth (cerebral palsy) and one child's parents were concerned about their child (hypothyroidism).
ASQ for 30-month-old children
In the 30th month, none of the variables were significantly associated with the scores (Table 1).
Regarding open-ended questions, all 30-month-old children had good hearing and vision, spoke understandably, and walked like others. One family had a history of trouble in a previous birth (cerebral palsy) and 3 children's parents were concerned about their child (hypothyroidism, hyperactivity, and obesity).
ASQ for 33-month-old children
In the 33rd month, none of the maternal and neonatal variables were associated significantly with the mean of scores at this age (Table 1).
In the open-ended questions, all 33-month-old children had good hearing and vision. One child's parents believed that their child did not talk and walk like peers and did not speak understandably. This child had lower ASQ scores in the areas of communication and gross motor. One family had a history of trouble in a previous birth (cerebral palsy) and 5 children's parents were concerned about their child (hypothyroidism, hyperactivity, motor retardation, and obesity).
ASQ for 36-month-old children
In the 36th month, none of the maternal and neonatal variables had significant correlations with neurodevelopmental status (Table 1). At this age, none of the variables related to asphyxia, including the 5th minute Apgar score, blood pH, base deficit and bicarbonate level, neonatal resuscitation (PPV, heart massage, intubation), and seizures, were associated significantly with neurodevelopmental status (Table 2).
Regarding the open-ended questions, all 36-month-old children had good hearing and vision. One child's parents said that their child did not talk and walk like peers and did not speak understandably. This child had lower ASQ scores in communication and gross motor areas. One family had a history of trouble in a previous birth (cerebral palsy) and 5 children's parents were concerned about their child (hypothyroidism, motor retardation, hyperactivity, and obesity).
ASQ for 42-month-old-children
In the 42nd month, none of the maternal and neonatal variables and variables related to asphyxia was associated significantly with neurodevelopmental status (Table 1 and 2).
In the open-ended questions, all 42-month-old children were reported to have good hearing and vision. Three children did not talk like peers (17.6%) 2 of which had lower ASQ scores in the communication area. One child did not speak understandably and this child also had lower ASQ scores in the area of communication. Two parents believed that their child did not walk like peers (11.8%) and these two had low scores in the gross motor area. Two families had a history of trouble in a previous birth (cerebral palsy) and 9 children's parents were concerned about their child (stuttering, hypothyroidism, leg pain, motor retardation, hyperactivity, and obesity).
ASQ for 48-month-old children
At this age, none of the maternal and neonatal variables and variables related to asphyxia had significant correlations with neurodevelopmental status (Table 1 and 2).
Among the open-ended questions, all 48-month-old children had good hearing and vision. Three did not talk like peers (25%) 2 of which had lower ASQ scores in the area of communication. One child, who had lower ASQ scores in the area of communication, did not speak understandably. Two children did not walk like others (16.7%) and these two had low scores in the gross motor area. One family had a history of trouble in a previous birth (cerebral palsy) and 6 children's parents were concerned about their child (stuttering, leg pain, motor retardation, noisiness, and obesity).
ASQ for 54-month-old children
At this stage, none of the maternal, neonatal, and asphyxia-related variables were associated significantly with neurodevelopmental status (Table 1 and 2).
Among the open questions, all 54-month-old children had good hearing and sight. Two children did not talk like peers (7.4%) both of whom had lower ASQ scores in communication. One child did not speak understandably and this child had lower ASQ scores in the area of communication. Two children did not walk like peers (15.4%) and these two had low scores in the gross motor area. One family had a history of trouble in a previous birth (cerebral palsy) and 4 children's parents were concerned about their child (hyperactivity, obesity, motor retardation, and improving stuttering).
ASQ for 60-month-old children
At the 60th month, none of the maternal and neonatal variables were significantly associated with neurodevelopmental status (Table 1). At this age, the 5th minute Apgar score (P = 0.044) and resuscitation by heart massage (P < 0.001) were significantly associated with neurodevelopmental status. Resuscitation by heart massage showed a significant correlation with all 5 ASQ studied areas (P < 0.001). Although endotracheal intubation was significantly associated with the area of problem-solving (P = 0.008), this association was not observed for 60 month mean scores (Table 2).
With the 60th month evaluation, the association between asphyxia-related variables and the 5 neurodevelopmental areas were studied (Table 3). Mean scores for the neurodevelopmental areas at 24 months were significantly associated with scores at 60 months (P = 0.013).
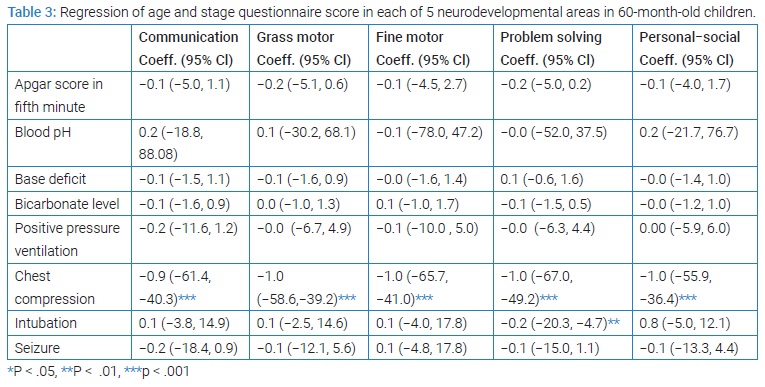
In addition, to obtain causality, a total score for the end of five years was given to each child and mutually causal relationships for the two groups were analyzed: one group with the neurodevelopmental disorder (2 patients) and another without the disorder. The analysis of factors showed that abnormal CT, EEG abnormalities and low Apgar scores were effective factors. To ensure potential confounding factors and remove them, the linear regression model was used, and finally, the only effective factor was a low 5th minute Apgar score (P = 0.029).
Concerning the open-ended questions, all 60-month-old children had good hearing and vision. One child, who had a lower ASQ score in the area of communication, did not talk like peers. One child did not walk like others and this child had low scores in the gross motor area. One family had a history of trouble in a previous birth (cerebral palsy) and 2 children's parents were concerned about their child (hyperactivity and motor retardation).
Discussion
About 15% to 20% of infants born with hypoxic-ischemic encephalopathy die within a short time and 25% of surviving children suffer from permanent neurodevelopmental problems [2]. In our earlier study in 2012, (3 of 30) neonates born with asphyxia died (10%) [13]. In other studies, the severity of encephalopathy was associated with mortality and severe disability [15,16]. Except for cerebral palsy, neurodevelopmental disabilities occur in 45% of term neonates with asphyxia and different degrees of neurological adverse effects may happen in motor, cognitive, memory, language, learning, and behavioral areas [2,5,12]. Despite our previous study results that reported 10.5% of 6-month-old infants, 14.3% of 12 month and 18-month-old infants, and 5.3% of 24-month-old infants showed a delay in the gross motor area [13], in the present survey following the previous cohort, 2 children (8%) had lower than cutoff scores in communication, gross motor, fine motor, problem solving and personal-social areas. In both of these children, blood pH at the first hour of birth was over 7. Their base deficits were -17.7 and -10. Bicarbonate levels were 11.2 and 13 respectively. They had 5th minute Apgar scores of 5 and 2, and both of them were resuscitated by the PPV. Chest compression was performed for one of them [13].
There is no consensus on the definition of fetal hypoxia-ischemia based on blood pH lower than 7 while some studies had reported ischemic injury with a pH higher than 7 [17–19]. In the present study, a significant association between blood pH and neurodevelopmental status was not found. However, in other studies, seizures and other neurological problems were more common at < 7 pH [20]. In our survey in 2012 as well, one of the neonates that died had pH < 7 though a significant correlation was not shown between pH < 7 and death outcome [13].
According to some reports, biochemical and clinical variables such as Apgar score and umbilical artery blood gases show limited value in future neurodevelopmental status prediction [2]. However, some studies have revealed that low umbilical pH is associated with infant mortality and morbidity [21,22]. However, in our previous assessment in 2011, a significant association between pH and ASQ scores was not found [13].
In the present survey, there was a significant relationship between resuscitation by heart massage and neurodevelopmental disorders in all ASQ areas at the ages of 24 months and 60 months. Also at the age of 60 months, 5th minute Apgar scores had a significant association with neurodevelopmental status (P = 0.029) and there was a relationship between intubation and neurodevelopmental status though only in the problem-solving area. This is in line with Handel et al. [23], which revealed that cognitive ability and school performance in children with moderate encephalopathy (without cerebral palsy) may face problems.
Although asphyxia is an important cause of neonatal encephalopathy, some maternal and neonatal factors influence neurodevelopmental problems [24]. Our findings showed no significant association between neurodevelopment status and the variables of sex, maternal age, gestational age, birth weight, gravidity, and meconium staining; delivery type showed a correlation but only at the age of 24 months. In other studies, being male was mentioned as a risk factor for asphyxia and 74.1% of the patients in our survey were boys [7]. According to other studies, children born with asphyxia are prone to behavioral disorders such as Attention Deficit Hyperactivity Disorder (ADHD), anxiety, depression, and social problems [23]. In our survey, parents of 4 children were concerned about their child’s hyperactivity which should be evaluated in the rest of this cohort.
In the present study, 7 mothers had disorders during pregnancy, including low count platelets and Glanzmann, alpha thalassemia, asthma, diabetes, hypothyroidism, and hypertension which have been reported in other studies as maternal risk factors of asphyxia [7,23].
Conclusion
Given that asphyxia is a significant cause of encephalopathy followed by permanent disabilities, in addition to preventive measures that need to be taken for the occurrence of asphyxia, medical examiners must be aware of the neurodevelopmental effects following asphyxia and with screening and early intervention to aid improvement of the final outcome. Also, we will continue to study the present cohort to follow-up on the next stages of these children's lives concerning long-term neurodevelopmental effects of asphyxia and cognitive and educational problems in children.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. 2008;199(6):587–595.
- Miller SP, Latal B, Clark H, Barnwell A, Glidden D, Barkovich AJ, et al. Clinical signs predict 30-month neurodevelopmental outcome after neonatal encephalopathy. Am J Obstet Gynecol. 2004;190(1):93–99.
- Wachtel EV, Hendricks-Muñoz KD. Current management of the infant who presents with neonatal encephalopathy. Curr Probl Pediatr Adolesc Health Care. 2011;41(5):132–53.
- Robertson CM, Perlman M. Follow-up of the term infant after hypoxic-ischemic encephalopathy. Paediatr Child Health. 2006;11(5):278–282.
- Al-Macki N, Miller SP, Hall N, Shevell M. The spectrum of abnormal neurologic outcomes subsequent to term intrapartum asphyxia. Pediatric Neurol. 2009;41(6):399–405.
- Perlman JM. Brain injury in the term infant. Seminars Perinatol. 2004..
- Okereafor A, Allsop J, Counsell SJ, Fitzpatrick J, Azzopardi D, Rutherford MA, et al. Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics. 2008;121(5):906–914.
- Obstetricians ACO, Gynecologists. ACOG committee opinion. Use and abuse of the Apgar score. Number 174, July 1996 (replaces No. 49, November 1986). Committee on Obstetric Practice and American Academy of Pediatrics: Committee on Fetus and Newborn. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 1996;54(3):303–305.
- MacLennan A. A template for defining a causal relation between acute intrapartum events and cerebral palsy: international consensus statement. BMJ. 1999;319(7216):1054–1059.
- Hankins GDV, Speer M. Defining the pathogenesis and pathophysiology of neonatal encephalopathy and cerebral palsy. Obstet Gynecol. 2003;102(3):628–636.
- Ishikawa T, Ogawa Y, Kanayama M, Wada Y. Long–term prognosis of asphyxiated full–term neonates with CNS complications. Brain Dev. 1987;9(1):48–53.
- Shevell MI. The “Bermuda triangle” of neonatal neurology: cerebral palsy, neonatal encephalopathy, and intrapartum asphyxia. Semin Pediatr Neurol. 2004;11(1):24–30.
- Keihani-Doust Z, Saeedi M, Esmaeilni T, Habibi M, Nazari SSH. Two-Year Follow-Up Study on Neurodevelopmental Outcomes After Term Intrapartum Asphyxia Using Age and Stages Questionnaire. J Child Neurol. 2013;28(12):1555–1561.
- Squires J, Bricker D. Ages & stages questionnaires [R], (ASQ-3 [TM]): A parent-completed child-monitoring system: ERIC; 2009.
- Viggedal G, Lundälv E, Carlsson G, Kjellmer I. Follow‐up into young adulthood after cardiopulmonary resuscitation in term and near‐term newborn infants. II. Neuropsychological consequences. Acta Paediatr. 2002;91(11):1218–1226.
- Robertson CM, Finer NN. Long-term follow-up of term neonates with perinatal asphyxia. Clin Perinatol. 1993;20(2):483–500.
- Yudkin PL, Johnson A, Clover LM, Murphy KW. Assessing the contribution of birth asphyxia to cerebral palsy in term singletons. Paediatr Perinat Epidemiol. 1995;9(2):156–170.
- Korst LM, Phelan JP, Wang YM, Martin GI, Ahn MO. Acute fetal asphyxia and permanent brain injury: a retrospective analysis of current indicators. J Matern Fetal Med. 1999;8(3):101–106.
- Leijser LM, Vein AA, Liauw L, Strauss T, Veen S, van Wezel-Meijler G. Prediction of short–term neurological outcome in full-term neonates with hypoxic-ischaemic encephalopathy based on combined use of electroencephalogram and neuro–imaging. Neuropediatrics. 2007;38(5):219–227.
- Sehdev HM, Stamilio DM, Macones GA, Graham E, Morgan MA. Predictive factors for neonatal morbidity in neonates with an umbilical arterial cord pH less than 7.00. Am J Obstet Gynecol. 1997;177(5):1030–1034.
- Fahey J, King TL. Intrauterine asphyxia: clinical implications for providers of intrapartum care. J Midwifery Womens Health. 2005;50(6):498–506.
- Johnston MV, Trescher WH, Ishida A, Nakajima W. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr Res. 2001;49(6):735–741.
- Van Handel M, Swaab H, de Vries LS, Jongmans MJ. Behavioral outcome in children with a history of neonatal encephalopathy following perinatal asphyxia. J Pediatr psychol. 2009:35(3):286–295.
- Badawi N, Kurinczuk JJ, Keogh JM, Alessandri LM, O'Sullivan F, Burton PR, et al. Intrapartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ. 1998;317(7172):1554–1558.
Keywords
Asphyxia; Neurodevelopmental status; Ages & stages questionnaires
Cite this article
Keihani-Doust Z, Saeedi M, Ashkanipour R, Kavyani R, Shariat M, Tehrani M. Neurodevelopmental status evaluation following up children born with term intrapartum asphyxia from 2 to 5 years of age. Glob J Pedia. 2022;1(1):1–7.
Copyright
© 2022 Maryam Saeedi. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).



