Dosage of Sulphur Dioxide in Jams and Jellies
Ionita AC;
Nicolescu TO;
Morosan E;
Gabriela Stanciu;
Nicolescu F;
Mititelu M;
-
Ionita AC: Department of Clinical Laboratory and Food Hygiene, Faculty of Pharmacy, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania.
-
Nicolescu TO: Department of Organic Chemistry, Faculty of Pharmacy, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania.
-
Morosan E: Department of Clinical Laboratory and Food Hygiene, Faculty of Pharmacy, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania.
-
Gabriela Stanciu: Department of Chemistry and Chemical Engineering, Ovidius University of Constanta, Constanta, Romania.
-
Nicolescu F: Department of Toxicology, Faculty of Pharmacy, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania.
-
Mititelu M: Department of Clinical Laboratory and Food Hygiene, Faculty of Pharmacy, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania.
-
May 06, 2022 |
-
Volume: 1 |
-
Issue: 1 |
-
Views: 3956 |
-
Downloads: 1285 |
Abstract
Sulphur Dioxide (SO2), labelled E220 on food packaging, is used as a preservative for certain dried fruits, canned vegetables and fruits, natural juices and in wine production, as an antimicrobial and antioxidant agent. SO2 present in small amounts of food is tolerated by most people, but in some people it can cause allergic reactions or other side effects, such as headaches. In the experimental part of the paper were analysed seven samples of jam and six samples of jelly from different fruits sold on the Romanian market in order to dose the total sulphur dioxide content. The experimental results showed high concentrations of SO2 above the maximum limits allowed in all the jam samples analysed and in five of the six jelly samples studied. The highest concentration of SO2 was recorded in bitter cherries jam (166.38 mg/kg) and cherry jelly (142.04 mg/kg), well above the maximum allowed limits (by over 3, respectively 2 times higher).
Introduction
Sulphur dioxide (SO2) and sulphites are used as preservatives, having bacteriostatic (prevents the proliferation of bacteria) and antifungal (destroying fungal spores). SO2 can be considered a universal antiseptic that acts on all microorganisms, but some yeast is more resistant than lactic or acetic bacteria. The most active form of microbicide is un-dissociated sulphuric acid [1,2].
In the food industry, a large part of fruits and vegetables subjected to dehydration are pre-treated with SO2 in order to preserve colour and flavour, as well as to reduce the loss of ascorbic acid and carotene. Also, with the help of SO2 can be avoided the phenomenon of mould of fruits that are kept at low temperatures, fresh. It preserves the unaltered colour of the wine and the cut fruits, their aroma and protects the carotene and vitamin C existing in them [3,4]. The use of sulphur dioxide for the preservation of fruit juices, syrups, concentrates and purees is widespread, especially in countries with warm climates where the cold storage network is insufficient. The SO2 content in the respective products varies depending on the condition of the raw material used, the initial microbial load, the storage time within relatively wide limits (0.035% to 0.15%) [1,3,4].
The action on the human body has been studied through numerous studies that have shown that SO2 and its derivatives in the quantities used in canning not be considered harmful to health. In the digestive tract and especially in contact with the mucous membranes, sulphuric acid is completely and rapidly oxidized and is eliminated as renal and digestive sulphates. SO2 gas is very irritating to the respiratory organs and eyes, causing bouts of coughing, bronchial catarrh and lung inflammation [5,6].
Used in higher doses (5 g/day) may cause abdominal pain and vomiting. Also, both sulphur dioxide and sulphites cause irritation of the gastric tract. They slow down the assimilation or even inactivate vitamin B1, by breaking the methylene bond of thiamine with the formation of pyrimidine and thiazole. If the pH is neutral, the process proceeds at high speed. May cause vitamin B1 deficiency [7,8].
Thus, cases of allergies (rhinitis, urticaria, atopic dermatitis, vertigo, dizziness, headache, asthma, vomiting) have been reported [9,10,11]. The toxicity limit is reached very quickly for people who are malnourished or weakened by a disease. Detoxification of the body can be best achieved by the participation of sulphitoxidase. The permissible WHO dose of SO2 and sulphites is a maximum of 0.7 mg/kg body weight/day (expressed as sulphur dioxide) [12,13].
In the experimental part of the paper were analysed seven samples of jam (from acacia flowers, bitter cherries, currants, cherries, blackberries, strawberries, raspberries) and six samples of jelly (plums, cherries, apricots, berries, peaches, jelly diet of cherry) marketed on the Romanian market in order to dose the total sulphur dioxide content.
Material and Methods
The optimized Monier-Williams method, which is a distillation-titration procedure (the sulphur dioxide distillation from complex matrixes followed by iodine titration) is most widely used for the analytical determination of sulphite in foods and beverages [14,15].
Chemicals
Starch solution 1%
Iodine solution 0.02N
Hydrochloric acid (HCl) 7%
Sample Preparation
The samples were homogenized avoiding unnecessary exposure to air. Weigh up to 10 g (± 1 mg) of each sample into a standard 250 mL test tube.
The receiving solution was prepared in a 250 mL Erlenmeyer flask, adding 75 mL of distilled water 10 drops of starch solution (0.6 mL) and 6 drops of iodine solution 0.02 N (0.25 mL). The receiving solution colour is light blue.
The samples were distilled according to the following parameters (set in a customizable method):
H2O (dilution water): 50 mL
HCl (7%): 30 mL
Distillation time: 6 minutes
Steam power: 50%
Set no automatic distillation residues discharge at the end of the distillation.
Were prepared some blanks with all the chemicals, without the sample.
The distillates were titrated with iodine solution 0.02 N in order to get the solution back to light blue.
It was found that using always the same amount of iodine reagent (6 drops) in the starting solution, titration is facilitated and accuracy is increased.
For record the mL of titration solution for the calculation was used the formula:

mL sample: Titrant volume used for sample
mL blank: Average of titrant volume used for blanks
MSO2: SO2 molecular weight (64.06 g/mol)
N: Normality titrant solution (0.02 N)
msample: Sample quantity (g)
All reagents used in the research were of analytical purity and have been purchased from Fluka Chemistry. Triplicate determinations were performed for each sample. Results are expressed as mean ± S.D (standard deviation) of triplicate analysis.
Statistical analysis of the collected data was performed using the open-source software (R Core Team 2019). Statistical significance was accepted for alpha-level 0.05. Because some samples from our study were too small for a classical approach, we applied a robust ANOVA version for comparing our datasets [16].
Results and Discussions
Following the dosing of SO2 in the jam samples, the following results were recorded in (Table 1). The analysis of the obtained results shows that all sweetness samples exceed the maximum limit allowed by the legislation in force (50 mg/kg). The highest amount of sulphur dioxide is in the samples of bitter cherry jam (166.38 mg/kg) and blackberry jam (115.31 mg/kg), and the lowest amount of SO2 is in the samples of currants jam (51.20 mg/kg) and raspberry jam (51.22 mg/kg).
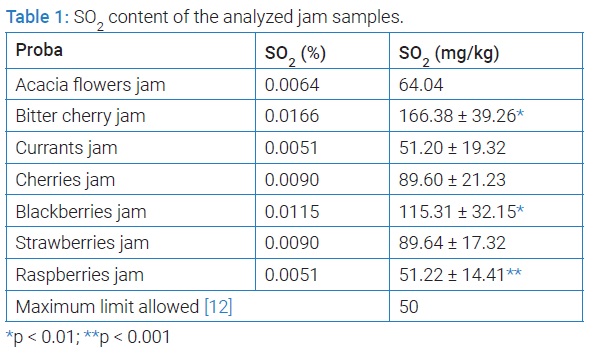
The experimental results obtained at the dosing of SO2 from the jelly samples analyzed are presented in (Table 2). The analysis of the obtained results shows that 5 of the 6 jam samples analyzed exceed the maximum limit allowed by the legislation in force (50 mg/kg). The highest amount of sulphur dioxide is in the jelly sample of cherries (142.04 mg/kg) and the lowest amount of sulphur dioxide is in the jelly sample of berries (25.61 mg/kg).
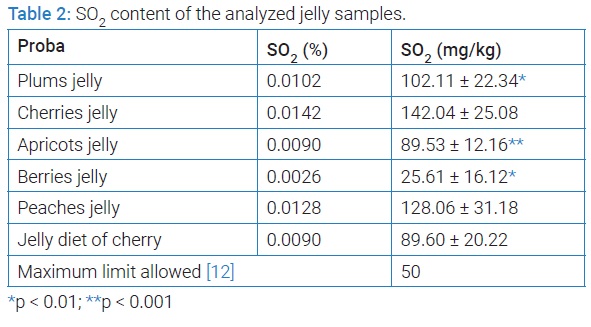
The experimental results indicate a tendency to use preservatives in concentrations higher than the maximum limits allowed in order to ensure the most stable stability of food preparations. Unfortunately, most food preservatives, especially synthetic preservatives, are not harmless, and failure to comply with food law can have serious consequences for consumers. In the case of sulfur dioxide, foods that contain high concentrations can cause gastric irritation, sensitivity to sulfites, allergic phenomena, sometimes fatal. Foods high in sulfur dioxide are contraindicated for people with allergy to sulfites and asthmatics. Consumption of these foods, especially those in which the maximum limits for sulfur dioxide are exceeded, can seriously damage the health of sensitive consumers [17,18].
Conclusions
According to the experimental results, an increased Sulphur Dioxide (SO2) content was found in the analyzed jam and jelly samples, sometimes over double the maximum allowed limits or even almost triple. Of the seven jam samples and six jelly samples taken, only one sample (berry jelly) had SO2 content below the maximum permitted limits. This indicates the need for more rigorous control over foods that contain additives that can cause health problems for consumers. There are regulations that aim to ensure the health of food consumption, but unfortunately manufacturers often use excess additives to increase the shelf life of perishable products endangering the health of the consumer.
For an adult body weighing 70 kg, the maximum amount of SO2 it can ingest is 49 mg SO2/day, which would require that adult to consume about 1 kg of jam or jelly per day not to exceed the maximum permissible concentration), which is unlikely. The situation is complicated when the amount of SO2 far exceeds the maximum allowable concentration. Consumption of these foods becomes a danger to human health, especially causing gastric diseases and allergic phenomena.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Weil ED, Sandler SR, Gernon M. Sulfur compounds kirk-othmer encyclopedia of chemical technology. John Wiley & Sons. 2006.
- U.S. Environmental protection agency. Reregistration eligibility decision document – inorganic sulfites. 2007.
- U.S. Food and Drug Administration (FDA). 21 Code of federal regulations. 2014;182:3862.
- World Health Organization. WHO technical report series No. 891. Sulfur dioxide and sulfites fifty first report of the joint FAO/WHO. Expert Committee on Food Additives. 1999.
- Timbo B, Koehler KM, Wolyniak C, Klontz KC. Sulfites--a food and drug administration review of recalls and reported adverse events. J Food Prot. 2004;67(8):1806–1811.
- Lien KW, Hsieh DPH, Huang HY, Wu CH, Ni SP, Ling MP. Food safety risk assessment for estimating dietary intake of sulfites in the Taiwanese population. Toxicology Reports. 2016;3:544–551.
- Taylor SL, Higley NA, Bush RK. Sulfites in foods: uses, analytical methods, residues, fate, exposure assessment, metabolism, toxicity, and hypersensitivity. Adv Food Res. 1986;30:1–76.
- Gokirmak M, Yildirim Z, Hasanoglu HC, Koksal N, Mehmet N. The role of oxidative stress in bronchoconstriction due to occupational sulfur dioxide exposure. Clin Chim Acta. 2003;331(1–2):119–126.
- O'Connor GT, Neas L, Vaughn B, Kattan M, Mitchell H, Crain EF, et al. Acute respiratory health effects of air pollution on children with asthma in US inner cities. J Allergy Clin Immunol. 2008;121(5):1133–1139.
- Li R, Meng Z, Xie J. Effects of sulfur dioxide on the expressions of EGF, EGFR, and COX-2 in airway of asthmatic rats. Arch Environ Contam Toxicol. 2008;54(4):748–757.
- Pressman P, Clemens R, Hayes W, Reddy C. Food additive safety. Toxicol Res Appl. 2017.
- Joint FAO/WHO expert committee on food additives. Safety evaluation of certain food additives and contaminants. 2009.
- Ordinul 438 din 2002 actualizat în 04.02.2011, Limitele maxime admise ale aditivilor alimentari.
- Pisoschi AM. Electroanalytical techniques for the determination of sulphite preservative: an editorial. Biochem Anal Biochem. 2014;3:e151.
- Kilmartin PA. Electrochemistry applied to the analysis of wine: A mini-review, Electrochemistry Communications. 2016;67:39–42.
- Mair P, Wilcox R. Robust Statistical Methods in R Using the WRS2 Package. Behav Res Methods. 2020;52(2):464–488.
- Franco R, Navarro G, Martínez-Pinilla E. Antioxidants versus food antioxidant additives and food preservatives. Antioxidants. 2019;8(11):542.
- ASCIA. Sulfite sensitivity Frequently Asked Questions (FAQ). 2021.
Keywords
Sulphur dioxide; Jams; Jellies
Cite this article
Ionita AC, Nicolescu TO, Morosan E, Stanciu G, Nicolescu F, Mititelu M. Dosage of sulphur dioxide in jams and jellies. J Nutr Food Sci Metab. 2022;1(1):1–5.
Copyright
© 2022 Gabriela Stanciu. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).



