Paroxysmal Dystonia and Multiple Sclerosis: Clinical Implication and Impact on Quality of Life
* Ben Mahmoud M;
Derbali H;
Messelmeni M;
Mansour M;
Zaouali J;
Mrissa R;
-
* Ben Mahmoud M: Department of Neurology, Military Hospital of Tunis, Tunisia.
-
Derbali H: Department of Neurology, Military Hospital of Tunis, Tunisia.
-
Messelmeni M: Department of Neurology, Military Hospital of Tunis, Tunisia.
-
Mansour M: Department of Neurology, Military Hospital of Tunis, Tunisia.
-
Zaouali J: Department of Neurology, Military Hospital of Tunis, Tunisia.
-
Mrissa R: Department of Neurology, Military Hospital of Tunis, Tunisia.
-
May 13, 2022 |
-
Volume: 1 |
-
Issue: 1 |
-
Views: 886 |
-
Downloads: 1284 |
Abstract
Paroxysmal Dystonia (PD) has been recognized as being part of the handicap caused by Multiple Sclerosis (MS).
Objective: Our study aimed to estimate the prevalence of PD related to MS in a Tunisian population, analyze its different statistical links and assess its impact on the physical and emotional functioning.
Methods: This research concerned 100 MS patients and PD was retained if diagnosed during the last 5 years or at time of assessment.
We did an assessment of patient’s general profile, MS clinical and MRI characteristics and impact on quality of life using SEP-59.
Results: The 11% had shown PD with a delay of 4.64 years. PD was positively correlated with female gender (p = 0.049) secondary progressive MS form (p = 0.039) and MRI lesions (thalamus (p = 0.01), capsulainterna (p = 0.01), cervical spinal cord (p = 0.04)). EDSS score had significantly increased in patients with PD (p = 0.002). Physical health, health perception and sleep dimensions were significantly worsen with PD.
Conclusion: Our findings seem to have important implications for clinical practice as it revealed implication of acute demyelinating lesions in the pathophysiology of PD in MS and the impact of PD on MS patients’ handicap.
Abbreviations
PD: Paroxysmal Dystonia; MS: Multiple Sclerosis; EDSS: Expanded Disability Status Scale; DMT: Disease Modifying Therapy; MRI: Magnetic Resonance Imaging; EEG: Electroencephalogram.
Introduction
Paroxysmal Dystonia (PD) has been recognized as being part of the handicap caused by Multiple Sclerosis (MS). The prevalence of PD varies from 11% to 19%, appearing during the course of the disease or even as the presenting symptom [1]. It still yet underestimated as semiology of these paroxysmal episodes raises several differential diagnoses. In 1958, Matthews et al., reported, for the first time, the entity “tonic spasm pain” to describe paroxysmal muscle contractions occurring in MS several times a day as involuntary, stereotyped movements, more often hemicorporal, with or without facial exposure [2]. The clinical characteristics differ from primary paroxysmal kinesigenic and non-kinesigenic dystonia [3]. Pathophysiological mechanisms are not well established, however some location of MS lesions related to PD have been reported [3–5]. Although PD are usually transient, lasting for few weeks with complete recovery, considering these symptoms as a relapse of MS still controversial and treatment strategies are not well established.
Objective
Our study aimed to estimate the prevalence of PD related to MS in a Tunisian population, analyze its different statistical links and assess its impact on the physical and emotional functioning.
Methods
The research consisted in a cross sectional, retrospective, descriptive and analytic study.
Participants and MS disease course
Our study concerned patients with MS, meeting the McDonalds 2017 criteria and followed in the department of neurology of HMPIT from 2017 to 2019.
All patients signed an informed consent. Patients that had experienced MS relapse in the preceding 3 months were excluded from the study.
We did an assessment of patient’s general profile, MS clinical characteristics (age of onset, duration, form, level of disability (Expanded Disability Status Scale (EDSS)), Disease Modifying Therapy (DMT).
The data was gathered from the medical history of patients, neurologic exam at time of consultation and from medical reports. All patients included in the study had Magnetic Resonance Imaging (MRI) performed less than five years ago.
We compared EDSS at MS onset and EDSS at time of assessment between patients with and without PD.
All patients underwent an interview that consisted on assessment of impact on quality of life using the French version “SEP-59” of the MSQOL-54 including 15 different items [6].
The final score of each dimension varied between 0 (worst score) and 100 (best score).
Brain MRI was acquired on a 1.5 Tesla MR equipment using a standard quadrature head coil. The protocol included a sagittal T2 weighted, an axial FLAIR sequence, and a sagittal T1 weighted sequence. Spine MRIs were acquired on the same equipment.
PD assessments
In the absence of clear diagnostic criteria for defining PD [3–5], PD was diagnosed by a neurologist when patients presented involuntary movement disorder characterized by sustained or intermittent muscle contractions causing abnormal, movements, postures, or both, lasting less than a minute often repetitive, occurring several times a day.
Diagnosis was made after excluding seizure episode [7].
Electroencephalogram (EEG) was performed for all patients with suspected PD and normal EEG recording during episode was obligatory to make the diagnosis.
PD was retained if reported during the last five years or at time of assessment.
We investigated:
- Delay of PD onset after initiation of MS
- Age of PD onset
- EDSS at PD onset
- The therapy received
Statistics
Categorical variables were summarized using frequency and percentage. The differences in PD between MS patients were stratified by demographic, clinical features, MRI findings, Disease Modifying Treatment (DMT), quality of life (SEP-59) using chi square test and conducted using IBM SPSS version 24. Significance level was p < 0.05.
Results
Descriptive
MS population: One hundred patients (22 women, 78 men) were enrolled in our study. Mean age was 36.71 years [18 years to 70 years]. Mean age at MS on set was 28.79 years [14 years to 55 years] and mean disease duration was 7.81, 86 patients had remittent MS form and 14 had progressive MS form.
Mean EDSS at MS onset was 2 and mean EDSS at assessment was 3.
In all, 66% patients were under disease modifying therapy (11% avonex, 13% betaferon, 9% rebif, 8% fingolimod, 22% natalizumab, 3% azathioprine).
PD investigation: The 11 patients had shown PD with a delay of 4.64 years after initiation of MS disease [3 to 10].
Mean age of PD onset was 35.64 ans [22 to 56] and mean EDSS score at PD onset was 4,091 [2 to 7].
Only 3 patients were treated for their PD; two patients received cambamazepine with improvement and the third one was under pregabaline after non-improvement under carabamazepine.
PD statistical analysis
Clinical links: PD was positively correlated with both female (p = 0.049) and with secondary progressive MS (p = 0.039).
Age of MS onset, EDSS at disease onset disease duration and DMT were not statistically different between subjects with and without PD (Table 1).
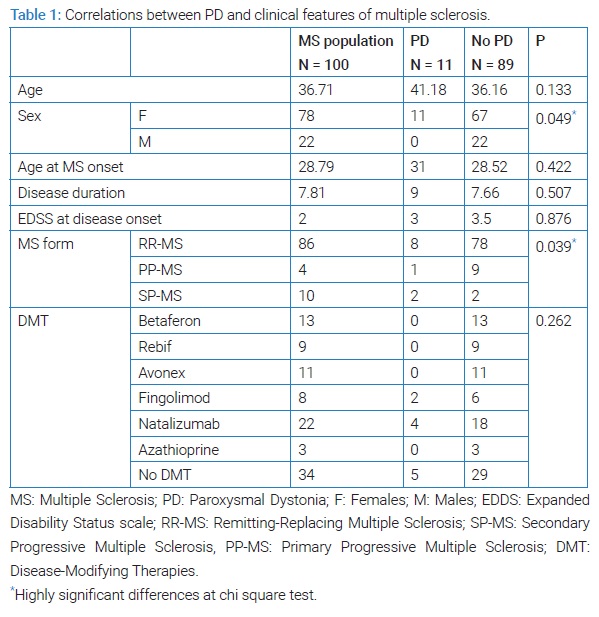
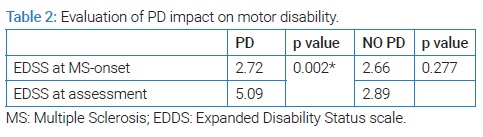
EDSS score had significantly increased in patients with PD (p = 0.002) compared to those without PD (p = 0.227) (Table 2).
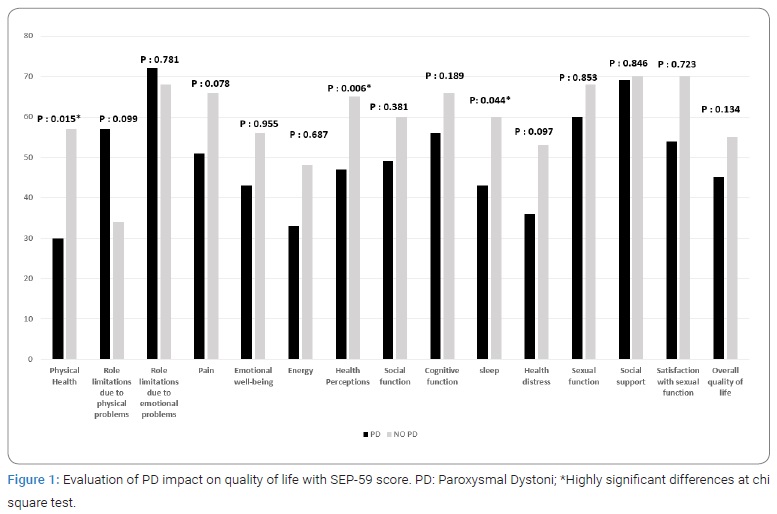
Physical health, health perception and sleep SEP-59 dimensions were significantly worsen with PD (p: 0.015, p: 0.006 and p: 0.044, respectively) (Figure 1).
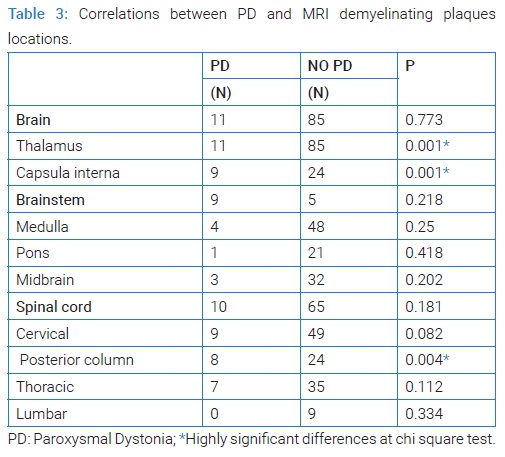
Radiological links: Number of patients with plaques in thalamus (p: 0.01), capsulainterna (p: 0.01) was statistically higher in PD group. In brainstem no differences were found between MS patients with PD and MS patients without PD.
In the spinal cord, we found a statistically significant difference between MS patients with and without PD (p = 0.04) (Table 3).
Discussion
Our finding that 11% of MS patients had experienced PD symptoms was similar to that reported from previous studies where the prevalence of PD in MS ranged from 11% to 19% [1].
In 1958, Matthews et al., reported for the first time, the entity “painful tonic spasms” to describe involuntary paroxysmal muscle contractions recurring several times a day that usually have a stereotypical course [2]. As shown in our study, no electroencephalographic abnormalities or loss of consciousness are observed during the attacks. However pain was not reported by all patients thus the term was abandoned and actually named «Paroxymal Dystonia».
Up until now, there are no clearly established diagnostic criteria for PD [3–5].
MS is the first cause of secondary PD [4]. PD is rarely inaugural and usually appeared with a delay that varied from 1 year to 10 years [3–5]. In our study, PD appeared during the course of MS with an average delay of 4.64 years, suggesting link with MS.
Similar to other studies, we found no significant differences between MS participants who reported PD and those who did not, based on age, age at MS onset disease duration, and DMT [3–5].
On the other hand, the sex ratio was statistically different between subjects with and without PD. This could be in accordance with the fact that MS patient were mostly female.
In our study, compared to non-PD group, SP-MS form was correlated with PD, suggesting that the prevalence of PD could increase when the disease has a progressive course.
But such a correlation was not found in many other studies as fortuitous association between MS and PD cannot be excluded. But our study suggested the possibility that PD in MS may results of a functional disability process or that there may be a causal relationship between the two conditions.
The pathophysiology of PD in MS is not clear and it was postulated that the symptoms result from ephaptic spreading of spontaneous discharges triggered by demyelinated axons at any level in a motor pathway [8].
In our study MRI showed a statistically significant link between PD and internal capsular, thalamic, and posterior cervical demyelinating plaques. Such associations have been reported by previous studies that demonstrated that acute demyelinating lesions in various locations, namely the spinal cord, contralateral cerebral peduncle, posterior limb of the internal capsule, thalamus, sub-thalamus thought to be responsible for PD [3–5]. But anatomic correlates are often difficult to establish, because of multiple co-occurring lesions and no enhancement after gadolinium.
Our findings seem to have important implications for clinical practice as it revealed a marked impact of PD on MS patients’ functioning and sense of well-being [9].
In fact, EDSS score statistically increased in our group patients with PD.
On the other hand, PD known to independently contribute to quality-of-life impairment [10,11], was associated in our PD group with stronger interference with activities of daily living particularly for Physical health, health perception and sleep as PD may also occur at night.
Although the negative impact of PD on quality of life, pharmalogical treatment is not systematic. It is conditioned by the degree of impact on daily activities.
Based on the possible implication of acute demyelinating lesions in the pathophysiology of PD in MS, some authors have demonstrated beneficial effect of steroid therapy [3]. However first line corticosteroids therapy is not justified as PD is not considered as authentic relapse of MS, requiring the use of other symptomatic treatments.
In our study, only three patients were treated for their PD. They received carbamazepine and pregabalin with good response.
In fact, PD may be managed with antiepileptic drugs including carbamazepine, phenytoin, gabapentin, pregabalin as well as intravenous infusions of lidocaine [3,4,12,13]. It has been hypothesized that the mechanism involved is an inhibition of voltage-activated sodium channels, and additional inhibition of voltage-gated calcium channels that explain cessation of ephaptic activation of neurons [5].
In other studies, treatment using acetazolamide or levetiracetam was reported as rapidly effective and safe strategy [13].
Botulinum toxin injections (repeated every three to four months) may also be used if a patient is suffering from localized, focal spasticity [13].
In summary, despite methodological limitations in our study, the fact that PD is prevalent in MS implies that it should be investigated in the clinical work-up of every MS patient. Although pathophysiological mechanisms are not well established, early recognition of PD and symptomatic treatment may significantly improve motor disability and quality of life.
Limitations
In accordance with previous studies, we classified patients with or without PD on the basis of self-reported PD during the past five years. Unfortunately, the duration of pain is rarely reported in other studies.
One limitation of this survey is the interval of five years used for both PD and imaging in order to optimize identification of radiological correlations. Another major limitation is the lack of information about PD severity, frequency and PD treatment duration.
In all patients presented with this movement disorder and had normal electroencephalogram, PD mimics, such as psychogenic dystonia as alternative diagnosis, may still be considered.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Brola W, Mitosek-Szewczyk K, Opara J. Symptomatology and pathogenesis of different types of pain in multiple sclerosis. Neurol Neurochir Pol. 2014;48(4):272–279.
- Matthews WB. Paroxysmal symptoms in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1975;38(6):617–623.
- El Otmani H, Benmansour Y, Araqi-Houssaini A, Benkirane N, Dany F, Rafai MA, et al. [Paroxysmal dystonia and multiple sclerosis]. Rev Neurol (Paris). 2014;170(2):119–123.
- Ciampi E, Uribe-San-Martín R, Godoy-Santín J, Cruz JP, Cárcamo-Rodríguez C, Juri C. Secondary paroxysmal dyskinesia in multiple sclerosis: Clinical-radiological features and treatment. Case report of seven patients. Mult Scler. 2017;23(13):1791–1795.
- Machado C, Amorim JM, Rodrigues M, Cerqueira J, Lourenço E, Pinho J. Paroxysmal dystonia as a manifestation of multiple sclerosis. Neurologist. 2015;19(5):132–134.
- Vernay D, Gerbaud L, Biolay S, Coste J, Debourse J, Aufauvre D, et al. [Quality of life and multiple sclerosis: validation of the french version of the self-questionnaire (SEP-59)]. Rev Neurol (Paris). 2000;156(3):247–263.
- Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VSC, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28(7):863–873.
- Ostermann PO, Westerberg CE. Paroxysmal attacks in multiple sclerosis. Brain. 1975;98(2):189–202.
- Bensmail D, Vermersch P. [Epidemiology and clinical assessment of spasticity in multiple sclerosis]. Neurol Rev. 2012;168:S45–S50.
- Chahraoui K, Bonin B, Couvreur G, Fromont A, Viegas N, Moreau T. [Subjective quality of life profile in patients with multiple sclerosis]. Neurol Rev. 2010;166(8–9):745–749.
- Pardessus V, Delattre S, Vermeersh P, Thévenon A. Study of the quality of life in 19 patients with multiple sclerosis. Ann Phys Rehabil Med. 1999;42(4):207–214.
- Pöllmann W, Feneberg W. Current management of pain associated with multiple sclerosis. CNS Drugs. 2008;22(4):291–324.
- Solaro C, Uccelli MM. Management of pain in multiple sclerosis: a pharmacological approach. Nat Rev Neurol. 2011;7(9):519–527.
Keywords
Paroxysmal dystonia; Quality of life; Multiple sclerosis
Cite this article
Ben Mahmoud M, Derbali H, Messelmeni M, Mansour M, Zaouali J, Mrissa R. Paroxysmal dystonia and multiple sclerosis: clinical implication and impact on quality of life. Ann Neur Res Stud. 2022;1(1):1–5.
Copyright
© 2022 Ben Mahmoud M. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).




