Green Hairy Tongue in Subcute Myelo-Optico-Neuropathy (SMON) and Boys with Twitching Muscles in Karachi and in Goroka with Subacute Sclerosing Pan-Encephalitisas (SSPE): 2 Clinical Findings That Led Research and Studies
* Toshiaki Takasu;
-
* Toshiaki Takasu: Nagaoka-nishi Hospital, Department of Neurology, Nagaoka-city, Niigata-prefecture, Japan; Nihon University, Tokyo, Japan.
-
Jul 04, 2022 |
-
Volume: 1 |
-
Issue: 1 |
-
Views: 1544 |
-
Downloads: 1384 |
Abstract
This paper exemplifies the clinical finding that led wide and long research and studies, shows the depth of green hairy tongue in Subacute myelo-optico-neuropathy (SMON) and looks for the actual risk of Subacute sclerosing pan-encephalitis (SSPE).
Part 1: Discovery of Green Hairy Tongue (GHT) in Subacute Myelo-Optico-Neuropathy (SMON) and research and studies raised by the discovery were reviewed. Forerun by attention to the strangely colored tongue dorsum, GHT was discovered. Green hairs were obtained by biopsy. Close connection of the green color of tongue and feces to SMON were demonstrated. Clioquinol was identified in green urine, feces and hair. The green substance proved to be clioquinol’s (3:1) chelate with ferric ion. The green hair was brought about by lingual hyper-keratosis. Close connection of clioquinol to hyper-keratosis was demonstrated. Lingual hypaer-keratosis was the sole visceral change of SMON. Close connection of clioquinol to SMON was verified. GHT in SMON symbolizes injury to life.
Part 2: Boys in Karachi with twitching muscles proved have suffered from Subacute Sclerosing Panencephalitis (SSPE). A total of 174 cases of SSPE were diagnosed at Civil Hospital Karachi between1982 and 1996. The inferred annual incidence of SSPE in Pakistan was 6.35 per 106 populations. Late measles sufferers occupied 65% of SSPE in Karachi. A total of 85 cases of SSPE were diagnosed at Goroka Base Hospital, Eastern Highlands Province (EHP), Papua New Guinea. The annual incidence of SSPE in EHP between 1997 and 1998 was 98 per 106 populations under 20 years. Risk factor analysis of SSPE in the 2 high incidence areas is needed.
Abbreviations
JMESC: Japan Ministry of Education, Science and Culture; JMHW: Japan Ministry of Health and Welfare; JMHL: Japan Ministry of Health and Labor; JMFA: Japan Ministry of Foreign Affairs; SMON, Subacute Myelo-Optico-Neuropathy; GHT: Green Hairy Tongue; GH: Green Hair; GT: Green Tongue; GF: Green Feces; GU: Green Urine; HT: Hairy Tongue; G: Green element of color; TUH: Tokyo University Hospital
Part 1
Introduction
Nobody will ever talk unless Takasu T now talks on GHT in SMON. It appeared in-around 1957 in Japan and disappeared in-around the end of 1970. Takasu T observed it in 1969 and 1970. It will perhaps never reappear again.
Subacute myelo-optico-neuropathy
SMON (1957~1970) was the short name of Subacute myelo-optico-neuropathy created by a group of 3 neurologists from Tokyo University, Tsubaki T, Toyokura Y and Tsukagoshi H; first presented in 1964 on Special symposium chaired by Kusui K, held by Japan Society of Internal Medicine. It very often was preceded by abdominal symptoms particularly diarrhea and pain. The disease appeared between 1957 and 1959 in 4 distant prefectures and then spread to other 2. The number of case totaled 68 before 1962. Its whole Japan annual incidence rose and rose from 41 in 1962 toward 1,552 in 1969. Viral infection was suspected by a leading physician as cause, basing on its familial or job-group or intra-hospital occurrences.
Several virologists reported responsible virus isolation from clinical materials. JMHW raised SMON Research Commission in the fall of 1969, to which chairman a senior virologist Reisaku Kono was appointed. Within 2 years after its start its members successfully elucidated the cause of SMON, which turned out to be the harmful action of clioquinol prescribed by doctor and taken by patient, therefore toxicity. For more comprehensive knowledge of clinical features of SMON readers can refer to the review by Toyokura Y and Takasu T [1].
Outline of Part 1
Forerun by attention to strangely colored tongue in SMON, discovery of GHT in SMON came up and streams of research and studies for cause of SMON arose.
First stream: Led by connection of GT and GF to SMON and their inclusion in the diagnosis guideline of SMON, discovery of GU came up, all these attained in pursuit of the green color in patient.
Second stream: Identification of green substance in SMON from green material was achieved by the isolation of clioquinol from GU followed by detection of (3:1) chelate of clioquinol with ferric ion in GU and GF, determination of high concentrations of zinc, iron and iodine and detection of clioquinol in GH; all these were attained in pursuit of the green substance in green materials of patient.
Third stream: Elucidation of pathogenesis of GH in SMON was achieved by discovery of lingual hyper-keratosis, its connection to clioquinol and its recognition as the sole visceral change of SMON, all these were attained in pursuit of GH in patient.
Fourth stream: Basing upon strongly suspected connection of clioquinol to SMON, sale and use of clioquinol containing medicines was governmentally suspended and, based upon verified connection by successive multidisciplinary studies, connection of clioquinol to SMON was concluded, all these attained in pursuit of the relationships of clioquinol to SMON in patient and to change equal to SMON in animals.
Attention and discovery
Attention to strangely colored tongue dorsum in SMON (1963–1969): Between 1963 and 1969 strangely colored tongue surfaces or furs became aware of by a few clinicians of their patients with SMON. Fifteen descriptions in some form were left by 8 observers. The color was described in 11 ways, namely as 5 pure color (black, green, brown, purple or white) or as 6 mixed color (black-blue, black-green, black-brown, yellow-green, black-purple, or gray-green). Among the 8, Tsubaki T (1963~), Toyokura Y (1966~), Takasu T (1966~), Takasaki H (1966~) and Shimada Y (1969) paid particular attention to it [2–11]. In spite of the mentioned above no medical scientific paper or no formal document open to public was left upon any GHT or GT or HT or tongue of patients with SMON for the 7 years between 1963 and 1969. During the period prior to 1970, no term of GT was ever in use by anyone. The discovery of GHT in a SMON patient changed the status.
Personally, so interested in the young woman’s black-green colored tongue demonstrated by Toyokura Y on November 2, 1966 Takasu T started research and some study late in 1966 lasting for the following 2 years and 10 months. In-between in 1968~1969 Toyokura Y had Takasu T shared 2 papers Toyokura Y procured on dog’s black tongue (but it’s not hairy tongue or black hairy tongue). On July 14, 1969 Takasu T searching literature on tongue came across the term ‘black hairy tongue in 2 books (Takasu T’s puzzled though over the description that the color of hair was mostly black but could be brown, red, green, yellow etc. and the dark purple or black one was called ‘black hairy tongue’.) and the term ‘pigmented hairy tongue’ in another. One of them wrote that HT looked like but was different from tongue fur. Another wrote that in black HT each hairy structure came into existence by elongation of the hyper-keratinized, most superficial layer of stratified squamous epithelium over filiform papilla of tongue dorsum, that the length of hair could be 15 millimeters and that HT was a pathological entity to be distinguished from tongue fur. The other book giving the term ‘pigmented HT’ said that dog’s black tongue due to deficiency of vitamin was different from the pigmented HT.
Discovery of green hairy tongue in SMON and harvesting green hairs from it (September1969):
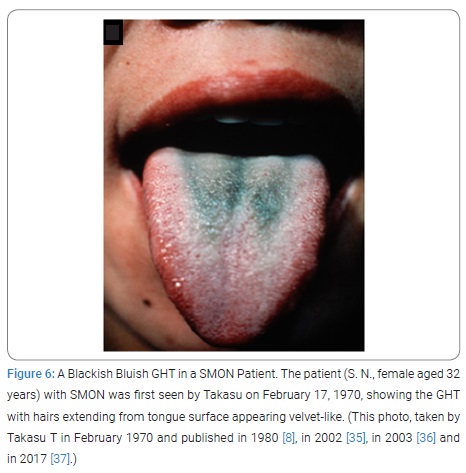
Takasu T first saw the patient, HN male aged 37 years, on September 13, 1969, who was admitted to TUH Neurology Ward because of aggravation of abdominal and nervous symptoms for 2 days before the admission. He was a case of SMON. So much induced by the doctor-in-charge’s saying “His tongue is really weird!” Takasu T saw the patient for the first time. At a glance his tongue was really weird. The tongue dorsum was in the depth widely dark-green colored giving a velvet-like appearance, the area surrounded by thick white furs [12] (Figure 1). On closer inspection of depth, groups of big, thick, several millimeter-long, tough-looking hairs were growing up dense [12]. Next moment Takasu T intuitively knew it was a HT and thought of a word “GHT” as its proper name (Figure 1). Toyokura Y hearing Takasu T’s reporting commented, “It’s Takasu T’s own, unique observation!.”
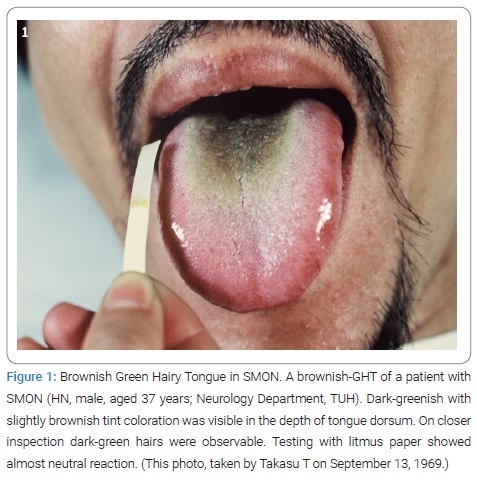
Since July 14, 1969 the term HT had been in Takasu T’s knowledge for 2 months. The readiness was all.
Because hairs were in front of Takasu T and within Takasu T’s reach Takasu T scraped off hairs from the 1st patient’s tongue to get a very small amount of GH. Ordinary microscopic examination in weak magnification of unfixed, unstained specimen had several GHs seen. Soon after the 1st, the 2nd patient with SMON, TS male aged 55 years, was introduced to Takasu T by another doctor Igata A, later collaborator of Takasu T, and examined by Takasu T to confirm another GHT (Figure 2.1). In the 2nd patient Takasu T performed cutting the hairs off with scissor in October, 1969 at the patient’s visited home to get nearly 1 cubic centimeter of GH at 1 time of cutting [12] (Figure 2.2). The harvested GH contained hairs of 5~6 millimeter in length. Stereo microscopic examination in 5 folds to 20 folds magnification of unfixed, unstained specimens revealed ample hairs giving a variety of pictures [12] (Figure 2.3–2.8). Most of the hairs themselves looked green [12].
Clearly GHT in SMON had 2 belongings, its color and its hair.
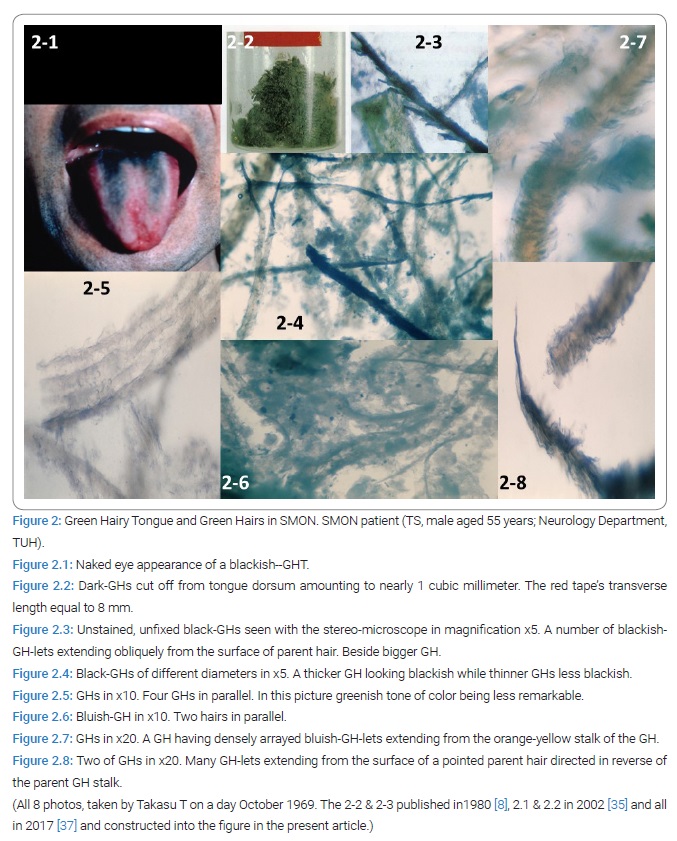
Green hairy tongue raised research and studies: The discovery and harvesting had mind of SMON scholars heavily thrust to deeper and materialistic studies for essentials of SMON. The excitement arising in Takasu T rapidly spread around. Takasu T began with trying extracting the green substance from it. Igata A began with offering GH to biochemist and microbiologist outside. Takasu T and Igata A was perhaps only the person who cut GH with scissor off from SMON patients harvesting them, in other words made GHT biopsy. The excitement continued until before the end of April 1970.
After the discovery of GHT heavy streams of research and studies for cause of SMON arose.
First Stream
Connection of Green Tongue and Green Feces to SMON and Their Inclusion in Diagnosis Guideline and Discovery of Green Urine (September 1969–June 1970)
Green color of tongue was common in SMON: Takasu T found out that green color of tongue was noted in the past clinical chart in 25/303 SMON patients (8.3%) registered at Neurology Department, TUH, between 1969 and 1970 and among them in 16/34 admitted to its ward (47.1%) [12]. Green color of tongue proved to be a frequently observable sign in SMON, its frequency depending upon the degree of searching [12].
Way of Finding out of green hairy tongue: GHT was all observed findings and generally subjectively symptomless. The great majority of them were discovered by chance by patients in a mirror or by careful clinician on complete general examination or intentionally by interested investigator. Deeper the interest in GHT was, the frequency of GHT rose. Rarely patients with GHT felt a feeling around throat.
Green element of color of tongue was specific to SMON: In another study by Takasu T and Igata A, the color, namely greenish color, of tongue was factorized in color element. The green element of color of tongue was described in the clinical chart in 14/55 admitted SMON patients (23.6%) at Neurology Ward, TUH, in contrast it was described in 0/98 admitted non-SMON with intestinal diseases (0%) in Internal Medicine Wards, TUH and another hospital in Tokyo, with highly significant difference (p < 0.0004) between SMON and non-SMON [12]. In contrast discolored tongue irrespective of its kind of color was noted in 16/55 SMON (29.1%) and in 7/98 non-SMON (7.1%), with less highly statistical difference [12]. Tongue fur was described in 27/55 in SMON (49.1%) and in 40/98 in non-SMON (40.8%), with no statistical significance [12]. This study result provided a strong evidence for the specificity of the green element of color of tongue to SMON [12].
Color factorization was the key to success. Factorization abstracted the green color element mixed with other color elements. Factorized element of color spoke for the insight of observers.
Lingual hyperkeratosis in autopsy case of SMON: In-between an autopsy case of SMON (TUH Chuken No. 25880-6, January 13, 1970) was preliminarily reported to Department of Neurology, by the pathologist-on-duty at TUH. The dead female patient (YY aged 45 years, A7795-69, DN-TUH) was hospitalized on April 22, 1969 and died of SMON on July 30 with a GT. A thick green colored tongue fur was continually noted until her death for the last 5 days. Microscopically hyper-keratsosis of the corneal stratum of mucosal tongue epithelium covering the filiform papillae was outstanding [12] (Figure 3.1 & 3.2). GH proved to be part of our body but not anything simply stuck or adhered onto the tongue surface. Takasu T and Igata A, convinced that the analysis of GH would be right way.
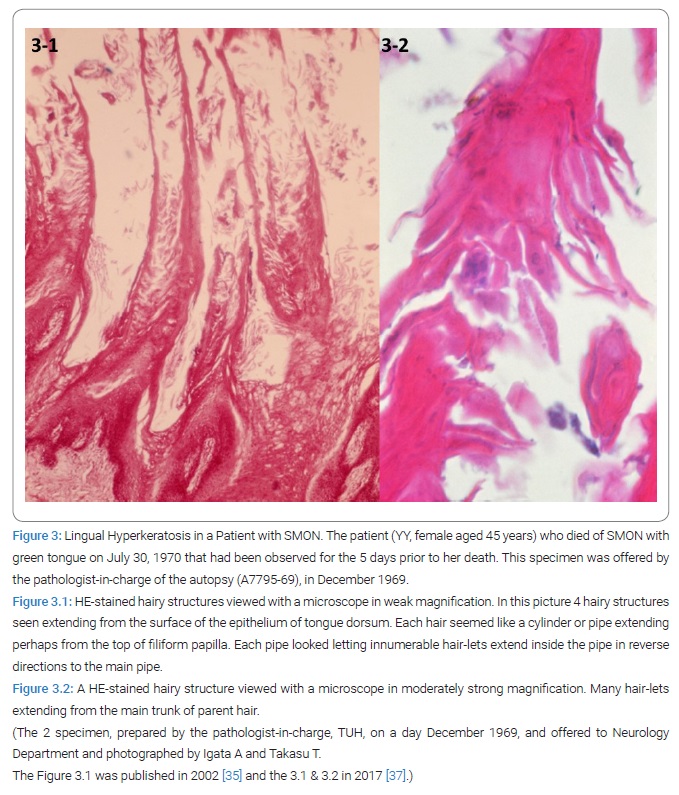
Close time connection of green color of tongue to SMON: Among the same series of patients as above, Takasu T found that GT had been noted at either SMON onset or its relapse in 8/8 patients (100%) for all of whom detailed clinical course diagram was available (Figure 4). This result suggested the close connection between GT and SMON [12].
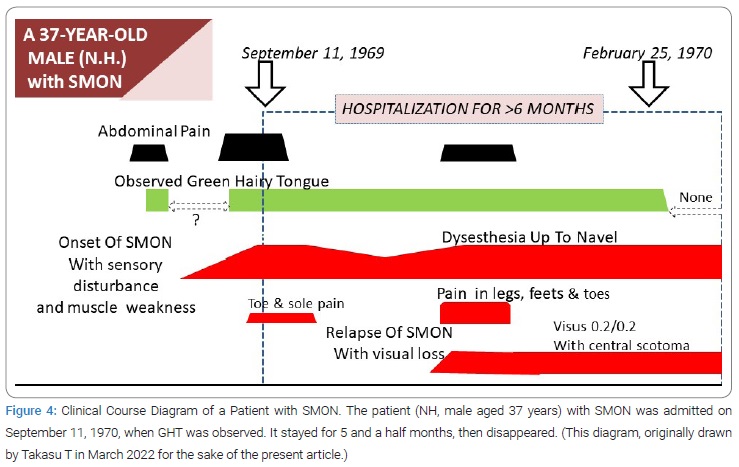
Creation of term green tongue: GT meant the tongue with green element of color, in other words greenish color of tongue. The term GT was created by Takasu T in preparation for the 1st presentation to public. It contained whole information about tongue that could be obtained from the past record prior to the discovery of GHT, where no description about hair was expected.
Presentations of Green Hairy Tongue to Public: In-between the 1st and 2nd presentations of GHT were performed on February 7, 1970 at the Japan Society of Neurology 32nd Kanto Areal Meeting and on February 14, 1970 at SMON research commission 2nd annual research meeting for fiscal 1969. The same content was presented on the 2 occasions by the names of Takasu T, Igata A & Toyokura Y. No response was obtained on the 1st presentation but strong response obtained on the 2nd , the chairman of the session Kenzo Kusui uttered “Indeed really so”.
Publication of original paper of green hairy tongue: The original paper was submitted on February 7, 1970 to Igaku-no-Ayumi i.e. J Clin Exp Med, received on February 17, 1970 and published on March 7, 1970 [12]. This was the 1st paper on the GHT in SMON or tongue fur in SMON or tongue in SMON. In that time 1970 the atmosphere surrounding SMON scholars was so much competitive that all scholars published their original work in weekly medical journals in Japanese, where they could expect earliest publication. Such journals were mostly with no English abstract. Such journals did not accept colored pictures in those days.
Green feces in SMON: Igata A, in pursuit of the green substance in SMON, discovered 6 patients with SMON who excreted, respectively, fairly thick green, green-black, fairy thick black-green, very black, or remarkably black-green colored feces during the time of onset (Figure 5), in the beginning 3 patients or in relapse in the other 3 [13].

On inquiry by Igata T 32/40 (80%) patients with SMON answered that they were themselves aware of the abnormal color of feces, mostly blackish green they catching it strange [13]. This work was received by Igaku-no-ayumi i.e. J Clin Exp Med on February 26, 1970 and published on March 21, 1970 by the names of Igata A, Takasu T &Toyokura Y [13]. This was the original paper on the green feces in SMON.
Green stool in Vioform taker in 1933: By the way, in the report in 1933 in JAMA on the treatment of amebiasis with iodochlorohydroxyquinoline (Vioform NNR), in its page 1,659, the authors wrote as untoward effects that “Usually the patient’s stools hardened after three or four days’ treatment, and some constipation was noted. The stools presented a characteristic oily green appearance. This may be used as an index of whether or not the patient takes the drug”. Therefore, in Igata A and collaborators’ report on GF in SMON [13], most important was the observation that the depth of feces’ green color fluctuated or changed along with appearance or relapse of symptoms of SMON.
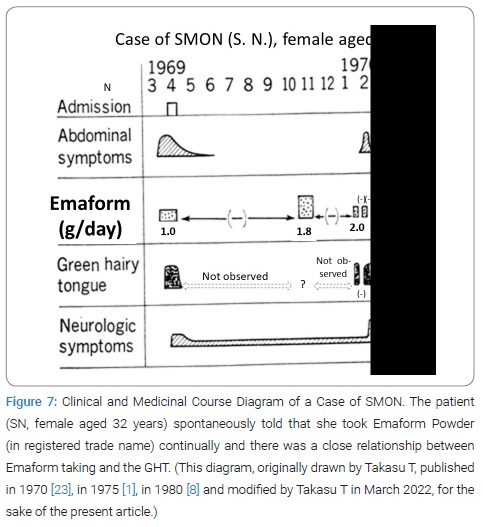
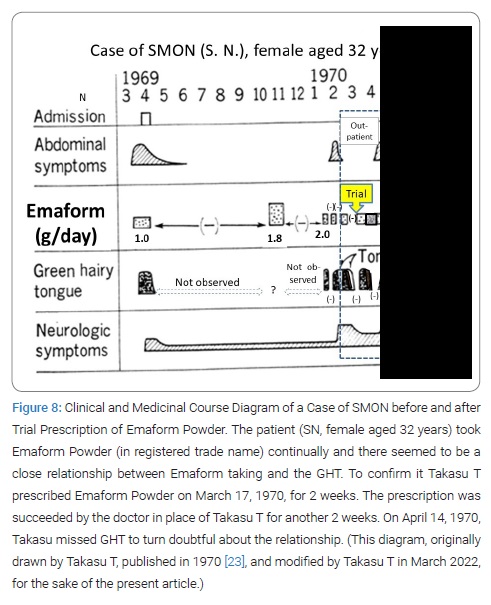
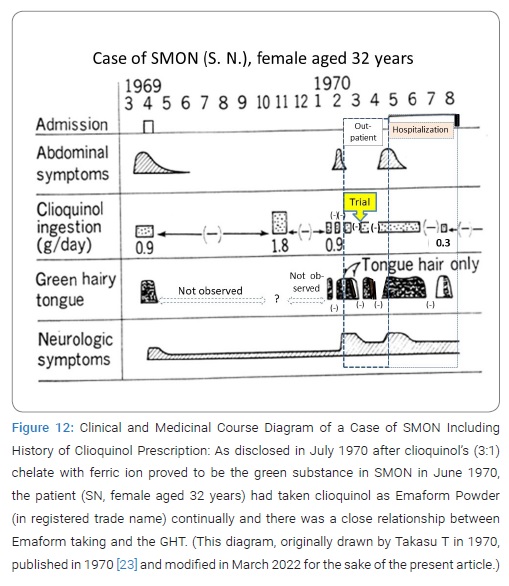
Interrupted trial suspension and resumption of Emaform Powder: The patient SN, female aged 32 years, on February 17, 1970, was first seen by Takasu T, showing GHT (Figure 6). Her SMON got started in April 1969. Her GT had appeared 3 times and disappeared 2 times during the 10 and half months since then (Figure 7). She spontaneously told that her tongue got green by taking the medicine that was Emaform Powder prescribed by the doctor outside introducer. She and her husband were aware of her GT appearing with the medicine and disappearing with substituting it with other medicine and reappearing with the medicine resumed. Conceiving the idea that Emaform Powder taking and GHT in SMON might be in relation, Takasu T let her continue to take the medicine in question prescribed by the doctor outside of which drug was unknown to Takasu T because its name was not mentioned in the letter of introduction. After the subsequent 4 weeks the actually observed close time relationship between Emaform Powder and her GHT (Figure 8) had Takasu T executed a trial plan prescribing Emaform Powder and its suspension as outpatient for the purpose of verifying the supposed relation for the weeks to come. In those days in 1970 clioquinol was a common medicine used for intestinal disorders including abdominal disorders in SMON and generally believed harmless. One gram of Emaform Powder contained 0.9 g clioquinol and 0.1 g carboxymethylcellulose added for medicinal dispersion. The trial plan, unfortunately, was interrupted on March, 31, 1970 after its 2 weeks’ progress because the executer Takasu T caught mumps and had to leave the duty as observer of the trial. The doctor in place of Takasu T on March 31 prescribed Emaform Powder for another 2 weeks. On coming back to the duty on April 14, 1970 Takasu T saw that no GT had been there for a time longer than a few days and became doubtful of the positive relationship’s hypothesis between Emaform Powder and GHT. Thus Takasu T’s image to Emaform Powder turned from probably guilty to probably innocent. The patient was hospitalized on the next day April 15, 1970 to leave Takasu T’s care of her as outpatient, so that the trial stopped.
Adoption of green tongue and green feces as reference item of SMON clinical diagnosis guideline: The SMON Research Commission ad hoc committee, chaired by Kenzo Kusui publicized the SMON clinical diagnosis guideline on May 8, 1970. It consisted of 2 cardinal signs and 6 other major signs, of which the (C) comprised the Greenish discoloration of Tongue (GT) and Feces (GF) [14].
Discovery of green urine: Igata A, Hasebe M & Tsuji T encountered 2 patients with SMON excreting GU [17]. The 1st case, female aged 73, the diagnosis of SMON was made because of her abdominal and nervous symptoms and observed GHT. On April 20, 1970, green substance was observed sticking to the inner wall of sustained urethral catheter, its finding by a nurse of the hospital who knew GT and GF in SMON, the knowledge given by Igata A’s lectures at the hospital. On May 20, stored urine in the jar itself got dark green (Figure 9), the green continued for a couple of week. To this patient oral 300 mg of ferrous fumarate daily had been prescribed from March 31 to April 13, succeeded by intravenous 40 mg of iron hydroxide 3 times a week. The 2nd case, female aged 49 years, hospitalized at the same hospital with the diagnosis of SMON. On 28th May, 1970 GU appeared, but disappeared in 3 days. The GU of the 2nd case was thinner in green. No iron preparation was administered to the 2nd case. In the 1st case, how and where the green substance was formed remained unclear as if the fortune a gift of nature.

In the 2nd case, where iron came from to urine was unclear. However, the importance of these 2 cases in the history of elucidation of the cause of SMON was of no doubt. They facilitated elucidation. This work was published in Nihon-Iji-Shinpou i. e. Jpn Med J September 19, 1970 [15], 45 days after the original paper on Clioquinol identification in GU by chemists was published.
Green hairy tongue was common in SMON (Succession): Kosaka K & Shimada Y reported that they saw GT in 40/71 (56.3 %) admitted patients with SMON in a hospital in Okayama Prefecture between 1969 and 1970 [16].
Green tongue frequency positively related to severity of SMON (June 1970): The studies in Okayama Prefecture at a hospital by Kosaka K & Shimada Y showed that GT was found in 22/50 patients with mild SMON (44%), 7/9 with moderate SMON (78%), 6/7 with severe (86%) and 5/5 who died (100%), with highly significant difference between 22/50 (44%) mild and 18/21 moderate, severe and died combined together (86%). Thus GT was observed in a frequency with a positive relationship to the severity of SMON [16].
Discovery of Green Hairy Tongue in SMON Did its 1st Role (June 1970): Discovery of GHT did its 1st role, namely connection of green element of color to SMON at this point of time (June 1970).
Second Stream
Identification of Green Substance in SMON from Material (October 1969–December 1970).
Isolation of clioquinol from green urine: Analytical chemists, Yoshioka M& Tamura Z, at Tokyo University Faculty of Pharmacy Medicinal Analytical Chemistry Department identified clioquinol in GU sample offered by Igata A in May to June 1970. Starting with 500 ml of GU, 116 mg of pale-yellow crystal was isolated that was proved to be clioquinol, i. e. 5-chloro-7-iode-8-hydroxy-quinoline [17] (Figure 10).
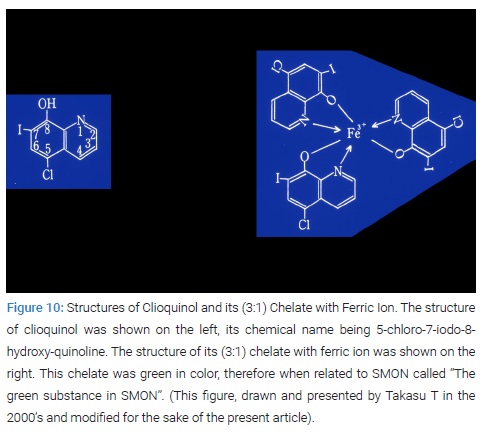
Detection of (3:1) chelate of clioquinol with ferric ion in green urine and green feces: Yoshioka M & Tamura Z obtained 1 mg of green residue from the 500 ml of GU in which Fe3+-clioquinol complex (i. e. (3:1) clioquinol chelate with ferric ion) was detected [17] (Figure 10).
Presentation of clioquinol to public: Yoshioka M and Tamura Z presented their result on June 30, 1970 at SMON Research Commission 1st Annual Research Meeting for Fiscal 1970.
Publication of clioquinol: Their paper was received by Igaku-no-Ayumi i. e. J Clin Exp Med on July 3, 1970, and published on August 15, 1970 [17]. This was the original paper of clioquinol in SMON.
By this time detection of the green pigment in GH was ongoing.
Determination of elevated concentrations of zinc and iron in green hair: Ikeda Y demonstrated elevated concentrations of zinc (13.860 μg/mg) and iron (1.218 μg/mg) in the dark GH sample (4.42 mg) of a SMON patient, female aged 73 years by atomic absorption spectrometry [18]. The result was reported on the SMON research commission annual research meeting on July 30, 1970.
Determination of high concentration of iodine in green hair: Kozuka H, Tsunoda N, Igata A and Takasu T demonstrated elevated concentrations of iodine (4.06 x 104 ppm, 1.44 x 104 ppm and 6.16 x 103 ppm) in 3 tongue GH samples from 5 patients with SMON (S.T., male aged 57; N.H., male aged 37; and 1 combined (T.K., female aged 78; G.S., female aged 58; and N.K., female aged 73) sample compared to 3.57 x 102 ppm of the brown tongue hair sample of a patient with SMON (S.S., female aged 32) by activation analysis. Their paper was received by Igaku-no-Ayumi i. e. J Clin Exp Med on September 8, 1970 and published on November 14, 1970 [19].
Detection of clioquinol in green hair: Imanari T and Tamura Z detected clioquinol in the extract of the GH biopsy sample of SMON patient. The extraction of clioquinol from the GH sample took more time than GU and GF. Imanari wrote that “the green substance in the GH was so tightly connected to tissue and its extraction with solvents was difficult to be done that direct verification could not be done. The green substance in the tongue hair could not be extracted with acetone, benzene or chloroform but with pyridine the color of the hair changed to get brown after 1 week. With this pyridine layer, the peak consistent with clioquinol was detected by gas chromatography”. Their paper was received by Igaku-no-Ayumi i. e. J Clin Exp Med on October 9, 1970 and published on December 5, 1970 [20].
Discovery of Green Hairy Tongue in SMON Did its 2nd Role: Discovery of GHT in SMON did its 2nd role that was identification of green substance in material of SMON at this point of time (December 1970).
Third Stream
Elucidation of Pathogenesis of Green Hair in SMON (December 1969 to March 1972).
Lingual hyperkeratosis in SMON recognized in an autopsy case: The pathologist-in-charge provisionally reported hyperkeratosis of the epidermis of tongue dorsum upon the filiform papillae in the end of 1969 [12]. Details were described above in the 1st stream of study section.
Close relationship between clioquinol and hyperkeratosis:
Ogawa K, Tsutsumi A & Motoi S reported that the most remarkable change in the viscera was para- or hyper-keratosis, the figure of so-called GHT [21]. They discovered a clear-cut relationship between the grade of lingual hyperkeratosis and the administered clioquinol until the death of patient [21]. Namely, among the 11 SMON patients who had taken clioquinol until 0 days to 4 days prior to death 6 patients showed hyperkeratosis in grade >++ and 2 patients hyperkeratosis in grade ++>+ and 3 patients in grade +/- or no hyperkeratosis, whereas among the 4 SMON patients who had taken clioquinol until longer than 35 days priot to death all 4 patients showed only ± or no hyperkeratosis.
Lingual hyperkeratosis as sole visceral change characteristic of SMON: Egashira Y, Chairman of Patohology Section of SMON Research Commission, concluded that lingual hyperkeratosis was the sole visceral change characteristic of SMON beside nervous system change on March 13, 1972 [22].
Discovery of Green Hairy Tongue Did its 3rd Role: GHT did its 3rd role that was elucidation of the pathogenesis of GH in SMON at this point of time (March 1972).
Fourth Stream
Connection of Clioquinol to SMON (July 1970–March 1972).
Start of connection of clioquinol to SMON: Known close connection of GHT to SMON and proved connection of green substance to clioquinol motivated 2 neurologists starting studies verifying it very early July, 1970. Very soon after the detection of clioquinol was reported Takasu T and Tsubaki T started studies independently, epidemiological works for clarifying the relation between SMON and clioquinol in Tokyo and in Niigata and Nagano Prefectures, respectively [23,24]. Takasu T had 3 reasons for the early start. Firstly Takasu T had concluded in the original paper that it was strongly suggested that GHT be related to the cause of SMON [12]. Secondly Takasu T as stated above had experienced a case suggesting the relationship between GHT and Emaform Powder 2 months before clioquinol loomed up (Figures 6 to 8,11 and 12). Thirdly Takasu T was firmly aware of the fact that non-SMON intestinal diseases did not show GT at all [12]. Therefore, if GT be shown up the case must not be non-SMON, namely must be SMON, as an example of contraposition in logic. Tsubaki T had 3 reasons. Firstly Tsubaki T in 1963 paid attention to the tongue often covered by green colored fur in the survey in Kushiro, Hokkaido [3]. Secondly, GT appeared in considerable rates in SMON, and thirdly, GT and GF were related to the up and down course of nervous symptoms of SMON [3].
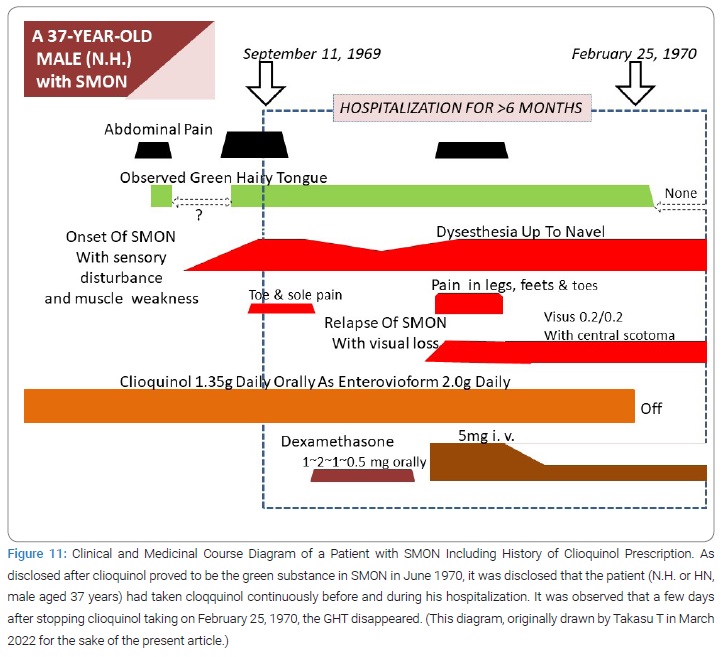
Discovery of Green Hairy Tongue Did Its 4th Role: The discovery of GHT did its 4th role that was mediation of clioquinol to SMON at this point of time (July 1970).
Clioquinol prescription in SMON: The medicinal history was disclosed in large numbers of SMON patient initially by 3 heads of group, Takasu T [23], Tsubaki T [24] and Yoshitake Y [25]. The patient (HN) had taken clioquinol as Enterovioform Powder® (in registered trade name) for longer than 5 months after his admission and for some time before his admission (Figure 11). The patient (SN), the very testee of the interrupted trial suspension and resumption of Emaform Powder by Takasu T, actually did take the medicine for only a few days after prescription and then stop taking it (Figure 12), as disclosed later by the patient to the doctor-in-charge at ward, where she stayed hospitalized from April 15 until August 1970, therefore the absence of GT on April 14,1970 was supporting the positive relationship between clioquinol taking and GT. Takasu T became known of the fact in July 1970 at last (Figure 12). She had stopped taking the medicine because the medicine prescribed March 31, 1970 by the doctor in place of Takasu T did not get yellow after a few days’ usage (the reason why remained unclear), apparently differing from the medicine prescribed by Takasu T that getting yellow in 2 weeks’ use. Thus Takasu T missed opportunities to observe the true effect of trial prescription of the Emaform Powder during the 2 weeks from March 31 to April 14, 1970 on her GHT. The patient must have had favor of Emaform Powder giving relief to her abdominal symptoms. Takasu T was as if looking at a shadow dancing played by the patient or at a night dream before dawn of the cause of SMON. A queer story, indeed it was. In an expanded study in TUH and 3 other hospitals, later performed, GT made a confirmed appearance from not-observed to observed in 23/28 times’ SMON onset (82.1%) and 43/78 times’ SMON relapse (55.1%), the difference being significant (p < 0.05) [23]. GT appeared more frequently at onset of SMON than at relapse of SMON [23].
Disappointment knowing green substance deriving from clioquinol: Hearing of the discovery of clioquinol in GU a number of concerned persons were disappointed. Tamura Z and Toyokura Y later in 1992 told of their disappointment on table talking [7]. Tamura Z said, “Yoshioka M reported to Igata A saying it’s a funny story. Igata A also was disappointed to hear that. Toyokura Y said I was also disappointed. Tamura Z, all of us disappointed (laugh)” [7]. The majority thought that clioquinol was a commonly used medicine for intestinal disorders including the abdominal disorders in SMON, so its usage in SMON being regarded as a matter of course and nothing any more. Yoshitake Y & Igata A, however, joined the study activity to relate clioquinol to SMON later [25]. For the purpose of the present paper focusing on GHT, no further details were inappropriate, so not described any longer.
Governmental suspension of clioquinol’ sale and use (September 1970): Strong suspect was raised after 1 month’s study. On August 7, 1970 Asahi News Paper reported on Tsubaki’s proposal to the Japanese government. On the 5th September 3 neurologists independently spoke on connection of clioquinol to SMON at Japan Society of Neurology 34th Kanto Areal Meeting. On joint discussion 6 leading neurologists admitted the positive role of taken clioquinol for SMON as a causative factor and 2 others did so only partially. Morizo Ishidate, representing the central drug matter council, played an important role in the decision making of the government. The Japanese Government took the measure to suspend the sale and the use of clioquinol or broxyquinoline containing drugs on September 8, 1970 [26]. Immediately after the measure new occurrence of SMON abruptly began to decrease and in another 3 months became nearly nul. Whole Japan monthly number by year of onset of SMON cases in 1970 were from January to December 126, 126, 137, 146, 178, 178, 177, 141, 37, 16, 4, 6 and unknown 2 [27].
Multidisciplinary studies (September 1970 to March 1972): Multidisciplinary studies were conducted by specialists. Epidemiologists recorded the whole Japan yearly and monthly occurrence of onset of SMON as of March 1972. The total number of SMON cases was 9,249, of which year at onset was known in 8,720 and unknown in 529. By year of onset the occurrence was 153 (1961 or before), 98 (1962), 66 (1963), 260 (1964), 451 (1965), 731 (1966), 1,452 (1967), 1,770 (1968), 2,340 (1969), 1,276 (1970), 23 (1971), and 0 (1972) [27]. They observed dose-response relationship between the dosage of clioquinol intake and the severity of some selected symptoms [26]. Pathologists studied 113 cases of typical SMON and established the nervous system and tongue changes characteristic of SMON [28]. Neuro-pathologists reviewed the nervous system changes and performed experimental modeling after human SMON upon clioquinol given dogs and rats [29–31]. Animal experimentalists demonstrated that about 20% to 28% of orally administered radio-labeled clioquinol was absorbed in mice [32]. Analytical chemists established a method enabling quantitative measurement of very little amount of unconjugated clioquinol showing clioquinol serum concentration of 10 μg/ml presumably being the required level for nervous tissue damage suppose it was kept for long time [33].
Public announcement by SMON research commission chairman Reisaku Kono (March 1972): The chairman of the SMON Research Commission Reisaku Kono made public announcement of the cause of SMON. “A tentative conclusion on etiology of SMON was announced in the general assembly of the commission on 13 March 1972 as follows: It is concluded that the great majority of patients who were clinically diagnosed as SMON were suffering from neurological distresses due to the intake of clioquinol. Thereafter, this conclusion has been strengthened further and contradictory facts have never appeared until now” [26]. The longest day to Kono, looking back of the day of announcement, was later recollected [34].
Green Hairy Tongue Originated Elucidation of Cause of SMON
GHT must have existed along with epidemic of SMON (1957–1970). A few clinicians paid attention to the strange color of tongue in SMON (1963–1969). Takasu T discovered GH of GHT (September 13, 1969). Takasu T and Igata A identified G color element as specific to SMON (publicized in February 1970). Wide research and studies arose. In 13 months clioquinol was identified in GU, GF and GH in this order. In 26 months lingual hyperkeratosis was identified as the sole visceral change of SMON, and the cause of SMON was elucidated. [35–37].
Honorary consultant: SMON Research Committee Chairman Kazuya Ando honored Toshiaki Takasu and Akihiro Igata as Honorary Consultant for the years 1991–1992.
Six why’s and provisional answer
1. Why was SMON research and studies for its cause activated promptly after 1969?
That’s because the sole color phenomenon in SMON had been left utterly helpless, but that was at last helped by the discovery of GHT in SMON. At this moment GHT was recognized as material but not as merely wired greenish color of tongue phenomenon. In other words GH as part of the body became available for chemical or microbiological analysis. Actually Takasu T began with GH trying extraction of green substance. Igata A started distributing GH to chemists and microbiologists outside. The activity was enforced by the tongue color now focused on its green element specific to SMON.
2. Why was term green hairy tongue not widely used?
That’s because all but a very few had never seen or heard the hair of tongue or the term GHT far from prompt understanding or how GH grows mysterious, and possibly for saving the face reasons in persons whose observation missed GH. Not seen meant not ready. The term GHT was properly used by Igata A in 1970 [13, 15], Toyokura Y in 1971, 1975 and 1993 [5-7], Ogawa K, Tsusumi A & Motoi S in 1971 [21], Iwashita H in 2001 [38], Inoue N in 2011 [39], Konagaya M in 2017 [41], Bareggi SR & Cornelli U in 2018 [40], and recently by Kuru Satoshi, Current chairman of SMON research committee, in 2021 [42], citing either or both of the original or 2nd paper except Iwashita H citing neither, although Toyokura Y admitted Kusui K not to use it in the SMON clinical diagnosis guideline. Kusui K understood in 1971 that GHT and GT were synonymous [14]. Ikeda Y in 1970 [18] and Kozuka H & Tsunoda N [19] in 1970 confounded GHT with tongue fur. Imanari [20] in 1970 used the word green fur instead of GH. Tamura Z in 2005 expressed the tongue in SMON as green hairy fur [43]. It was not used by Kono R [26] in 1975 who cited the original work of GHT stating that the fur of the tongue was colored green and wrote in 1981 [34] that Takasu T reported GT was seen in 7% to 47% of SMON patients, not seen in other diseases and was often accompanied by the development of the SMON’s abdominal and nervous symptoms of its own. Shiraki H in 1975 [29] wrote “Since the “green tongue” comprised an important clinical characteristic of “SMON”. Its pathology should briefly describe here, and this can be summarized as follows”. Shigematsu I in 1975 [27] did not mention GHT or GT.
3. Why was term green tongue widely used?
That’s mainly because it was simple as word and significant enough for practical use or ordinary medical clinical study. The term GT spoke for the insight of observers. Factorized green color element spoke for the insight of observers. Some of its users felt as if they were acquitted or relieved from their missing GH. Kosaka K & Shimada Y in 1970 [16] and Tsubaki T et al. in 1971 [3] cited Takasu’s 1st and 2nd or 1st paper, respectively, on GHT using GT instead of GHT. As a matter of fact it was often extendedly or extrapolatedly used, namely going back to the older times when the term “GT” did not exist, by many, even by SMON scholars like Kono R [34], Tsubaki T [24], or Takasaki H [11].
4. Why was green hairy tongue mixed with other color element?
Presumably that’s because other metals than iron like zinc or cupper might possibly be involved in chelation by clioquinol. The possibility was concreted by the diagram given in Kuru S’s review [42]. Clioquinol can interact with copper, zinc and iron with its metal binding capacity [40]. Iron and zinc concentrations were increased in GH of a SMON patient [18]. However, what color those metal chelates other than ferric iron chelate present is unclear. Homma Y et al. stated in Nippon Rinsho i. e. Jpn J Clin Med 1971;29:41-46 that the blue-green, gray-white, purple or yellow tongue fur might be involved by Serratia maroscems detected in 4/18 SMON patients.
5. Why was clioquinol detected in green hair so late?
That’s because GU and GF were excretions whereas GH was part of the body, in other words a tissue with histological structure. Among 3 kinds of material GH, GF and GU, GH was the earliest in discovery but the last in successful detection of clioquinol even by the identical group of researchers headed by Tamura Z. Yoshioka M & Tamura Z wrote that the green pigment in GH combined with high molecular substances could not easily be extracted with organic solvents [17]. Imanari T & Tamura Z wrote that clioquinol was not extracted from GH with acetone, benzene or chloroform but was extracted with pyridine only after 1 week’s immersion [20]. Takasu T independently only demonstrated the green substance in chloroform extracts of GU and GF of a patient by thin-layer chromatography and confirmed it’s different from biliverdin and from cyanomycin.
6. Why was green hairy tongue variously shaped?
Filiform papilla 0.7 mm to 3.0 mm in height is distributed on the whole tongue dorsum diffusely and densely [46]. However, actual GHT often localized in the depth (Figure 2.1 and 6) or in some instances forked on the median line in front (Figure 2.1 and 6). That’s presumably because tongue movement varies individually, often but not always active on the periphery than on the center, so that wearing-off effect on the epithelium by mechanical stress by tongue movement can differ individually.
Review of Behavior of Clioquinol in Tongue
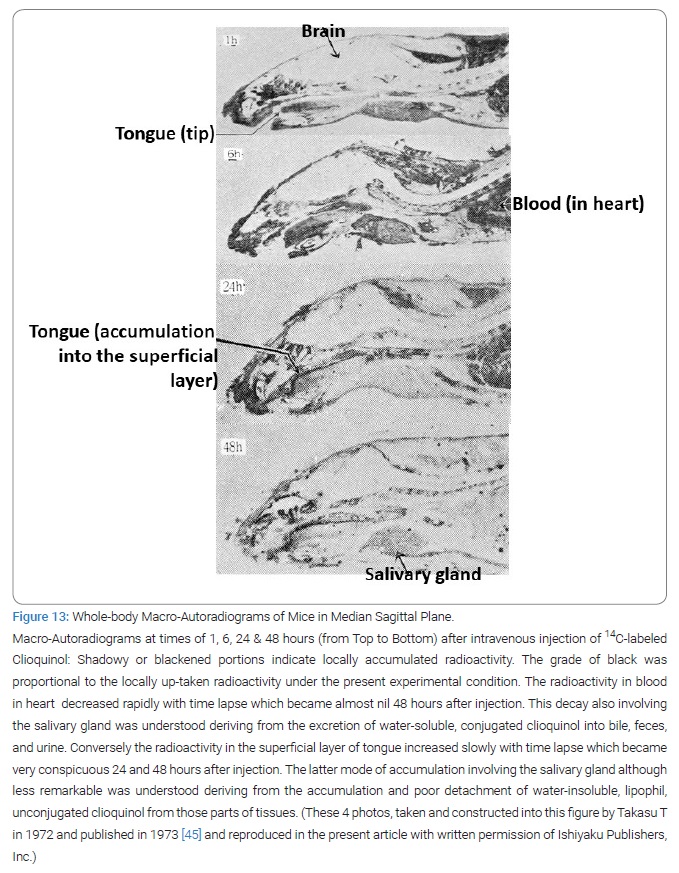
Inner route of clioquinol to superficial layer of tongue: After orally given at one time, 131I-clioquinol solved in olive oil had between 20% to 30% of its radioactivity was measured in the residual parts of the body outside the gastrointestinal tract and its contents at 2 hours after administration in mice [32,33]. Intravenously injected 14C-clioquinol accumulated in the superficial layer of tongue of mice at 24 hours and 48 hours after injection (Figure 13) [45]. Clioquinol was absorbed by serum albumin in blood [33].
Outer route of clioquinol to superficial layer of tongue: Orally crunched and chewed Enterovioform® Tablet (1 tablet containing 250 mg of clioquinol) or Emafrom® Tablet (1 tablet containing 100 mg of clioquinol) could not color the tongue surface either of human SMON or non-SMON testees while Strengthened-Mexaform® Tablet (1 tablet containing 200 mg of clioquinol and 20 mg entobex) could color it green. Entobexalone when touched to the tongue surface could reinforced the green in 4 non-SMON testees, whose tongue surface had got slightly green on crunch-and-chewing test with strengthened-Mexaform [44].
Chemical form in vivo: Tamura Z [33] understood that clioquinol in excretion was in conjugated form while clioquinolin tissue was in unconjugated form and in both forms in the generally circulating blood. Conjugated clioquinol soluble in water was excreted in bile from liver, then in feces and in urine from kidney. Lipophil free clioquinol could transfer into the nervous tissue [33]. To produce degeneration in the nervous system, perhaps it is necessary to maintain the concentration of free clioquinol in serum around 20 μg/ml for several days [33]. Free clioquinol can chelates ferric ion to make Fe3+-clioquinol complex that is green in color [33]. The (3:1) chelate of clioquinol with ferric ion connected to high-molecular compound tightly, resisting extraction [20].
Change in chemical form of metal by pre-existing clioquinol: The large amount of free clioquinol administered 24 hours in advance could affect the whole body distribution of intravenously injected 59Fe3+ or 65Zn2+ meaning the change in chemical form of that metal taking place, presumed by combination of clioquinol with that metallic ion [47]. Clioquinol is able to chelate and redistribute metals [40].
Metal increase in green hair in SMON: Iron, zinc and copper were increased in the GH of a SMON patient [18]. However, whether the relative presence of the individual metal in GH is always consistent or not is unknown. Furthermore, whether the relative presence of the individual metal in GH is the same as that in nerve cell is unknown. In other words, if GH indicates the presence of ferric iron chelate in nerve cell is obscure.
Interaction of unconjugated clioquinol to living cell constituent: In the nerve cell, clioquinol plays a role as an uncoupler of the oxidative phosphorylation in mitochondria in the presence of divalent metallic ion such as Mg2+, and such disturbances of electron transmission system may be the essential element of toxicity of clioquinol [33]. Clioquinol is considered to be a Metal Protein Attenuating Compound (MPAC) that acts as zinc, cupper and iron chelator [40]. As it is a mitochondrial toxin, clioquinol/Zn chelate has been considered to be the ultimate cause of SMON [40]. The (3:1) chelate of clioquinol with ferric ion firmly stuck to high molecular compound in GH to resist extraction with organic solvent and barely dissolved in pyridine after 1 week’s immersion [20]. Unconjugated clioquinol can cause lipid peroxide production in vivo [48].
Fate of lingual cell: Clioquinol caused hyper-keratosis [21].
Fate of unconjugated clioquinol in dead lingual epithelial cell: Dead cell of tongue epithelium cannot be easily lost by desquamation of individual cells but by wearing-off of clustered cells [49]. Weakened maximum tongue pressure in SMON [50] can contribute to the reduced wearing off in clusters of cornified lingual epithelium.
Review of Filiform Papilla of Human Tongue Epithelium and Keratin: Filiform papilla is slender, dense in the whole tongue dorsum and 0.7 mm to 3.0 mm in height [46]. No keratinization of mucosal epithelium takes place in the oral cavity except lingual filiform papillae [46]. Hair-type or acidic keratins are demonstrated exclusively in the human filiform papillae [51]. Keratin 36 was a specific marker of tongue filiform papillae [52]. Keratin and neurofilament belong to the intermediate filament of cytoskeleton in tongue epithelium and neuron [52,53], respectively. In SMON the lingual epithelium was in hyper-keratosis [12,21,22]. The unfixed and unstained, biopsied tongue hair in SMON was green on stereomicroscopy [12] and 14C-clioquinol was accumulated in the superficial layer of tongue of mice [45].
Pathogenesis of green hairy tongue as hypothesis: Unconjugated clioquinol arriving at the epithelial cells of tongue mucosa enters the cells, where it connects with its quinoline ring to Keratin 36 in the filiform papilla of human tongue resulting in the elongated thread-like cornified projection, namely tongue hair. This occurs because unconjugated clioquinol sticks to higher molecular compounds so tightly that clioquinol stays long in the cell, leading to the prolonged deprivation of ferric ion and other metals from the cell, which results in accelerated cell death namely accelerated cornification namely hardening of hair, resulting in reduced chance of wearing-off of hair in clusters and enhanced chance of elongation of hair. Decreased tongue strength caused by clioquinol contributes to the reduction of wearing-off. To maintain such elongated hair clioquinol must be supplied from outside to growing fresh hair that’s why the G of GH disappeared a few days after clioquinol taking suspended. The same, or similar, mechanism is expected in nerve cell as, or to, in tongue epithelial cell since both conditions GHT and SMON behaved very similarly, where neurofilament must play a similar role to Keratin 36 in lingual filiform papilla.
Summary of Study Result in Part 1
Discovery of green hairy tongue in SMON
GHT must have existed along with SMON between 1957 and 1970 for 14 years. But its strange color had been unnoticed before 1963 for 6 years and its hair unnoticed before September 1969 for as long as 12 years. It became noticed on September 13, 1970. GHT consisted of the green color phenomenon and the hair material.
Research and studies raised
Discovery of GHT raised four heavy streams of studies. GHT did its role at 4 respective points of time, when green urine was discovered (April to May 1969), when clioquinol was detected in the green tongue hair sample (December 1970), and when lingual hyper-keratosis caused by clioquinol was recognized as the sole characteristic visceral change of SMON (March 1972) and when studies connecting clioquinol to SMON got started (July 1970),
Essentials of green hairy tongue
The essentials of GHT and SMON were the injury of life by taken clioquinol, both being closely related to each other in clinical manifestation and presumably in underlying pathogenetic mechanism, namely metal chelation by clioquinol. In other words, GHT and SMON are different expressions of life injury due to clioquinol action.
Meaning of green hairy tongue
GHT symbolizes SMON as a model of the iatrogenic disease. GHT in SMON was the signal that life was suffering.
Conclusion of Part 1
Knowledge delighted us. Knowledge righted us. Clinical observation sometimes opened doors for new knowledge. Human trust sometimes had obstacles surmounted. Truth could be suggested by amateur. However, all above were not enough for scientific verification. Nature gave once.
Green hairy tongue was specifically reviewed and deeply discussed for the 1st time.
Part 2
Boys with twitching musclesin Karachi and in Goroka with subacute sclerosing pan-encephalitis.
Abbreviations
SSPE: Subacute sclerosing pan-encephalitis;EEG: electroencephalography; CSF: cerebrospinal fluid; PBMC: peripheral blood mononuclear cell; HI: hemagglutination inhibition; NT: neutralization titration; JE: Japanese encephalitis; WNE: West Nile encephalitis; JEV: Japanese encephalitis virus; WNV: West Nile encephalitis virus; DMC: Dow Medical College; CHK: Civil Hosital Karachi; JPMA: Journal of Pakistan Medical Association; PNG: Papua New Guinea; EHP: Eastern Highlands Province; GBH: Goroka Base Hospital; PNGIMR: Papua New Guinea Institute of Medical Research
Introduction
The 2nd clinical finding that started research and studies lasting for the next 16 years in Karachi and 6 years in Goroka was the “young boys with twitching muscles in Karachi”, discovered by a Pakistani neurologist Akhtar Ahmed. Between 1972 and 1978 he often saw a SSPE-like encephalopathy appearing around him. Diagnosis was aided in selected cases by the periodic synchronous discharges repeatedly recorded by an 8-cahnnel EEG which were barely discernible. His observation was published in Journal of Pakistan Medical Association in November 1980 as Case report headed by “Sub-acute sclerosing panencephalitis a report of seven cases” [54]. All 7 cases had intellectual deterioration, speech difficulty, gait difficulty and myoclonic jerks. The finding by Akhtar of those young boys with twitching muscles and their worried parents had a continuing stream of research and studies started.
Subacute sclerosing pan-encephalitis
SSPE was a rare disease in childhood in Japan or USA or European countries. The great majority of patients had a history of childhood measles. Once it started mental and physical deterioration progressed without major remission until death in several years in almost all the cases if no treatment with interferon or Isoprinosine or Ribavirin was conducted as was usual in Pakistan in those years 1981 to 1996 and in Papua New Guinea in 1996 to 2000. Boys are affected more than girls. Muscle twitching due to myoclonic jerk or fit was a common sign of the disease.
Subacute Sclerosing Pan-Encephalitis in Karachi (1981~1996)
Human relation: It was true that the human relations between Akhtar Ahmed and the Japanese neurosurgeon Kintomo Takakura, between Takakura and his colleague neurologist at TUH Toshiaki Takasu and between Takasu and Former Chairman of SMON Research Commission Reisaku Kono, were really essential for the purpose of realizing the international collaborative study between Japan and Pakistan.
Karachi in 1981 to 1996: Karachi was located in the west end of Indian Subcontinent on the seacoast of Indian Sea in low latitude with subtropical climate. It was populated between 60 million to100 million during the years 1981 to 1996. The population increase rate was 31 per thousand in 1980 to 1984, the infantile death rate 84 in 1979 urban and 153 in 1988 per 1,000 birth and the measles vaccination coverage 53% of children under 5 years in 1984 [55–57]. Education unevenly distributed, the literacy rate was 25% in the 1980’s in the whole Pakistan [57].
Who was Akhtar Ahmed?
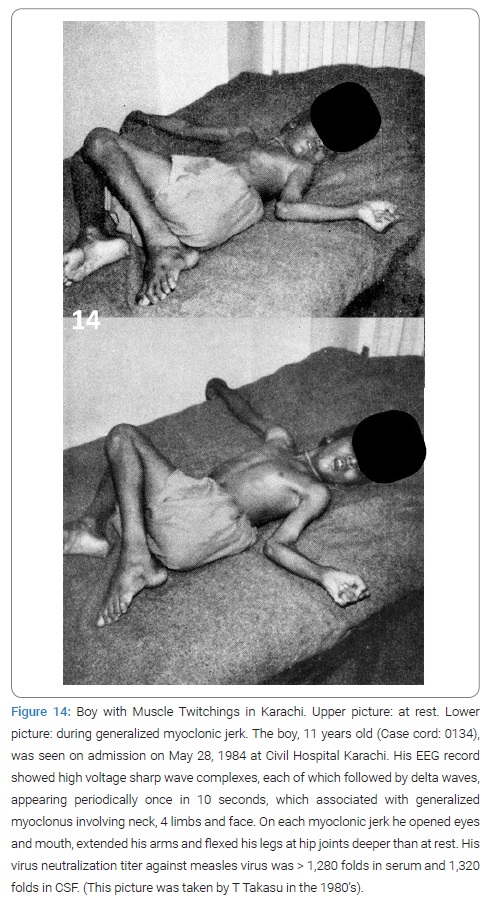
Born in South India, he moved to Karachi in 1947, graduated from Dow Medical College in Karachi, in 1957, with top records. He went over to UK where he was trained in neurology then to Cleveland, USA. Afterwards in 1978 he was awarded F. R. C. P. (Edinburgh) in neurology and became recognized as the 1st authorized neurologist in Pakistan. He was appointed to the first Head of Neurology Department of Dow Medical College and Civil Hospital Karachi that was established in 1974, as the 1st independent department of neurology in Pakistan. He must see so many young boys with twitching muscles appearing with their father or mother and their death in the hospital. His diagnosis of SSPE numbered 25 between 1974 and 1980. Later Takasu observed such boys more than once together with Akhtar (Figure 14). But his diagnosis was not supported by serology because of the lack of serological diagnostic facilities in Karachi in those times. No CT or no MRI was on the stage, either, at that time.
This situation was first conveyed to Takasu in 1979 by Akhtar. Takasu introduced Akhtar to Kono, at that time Director of the Central Laboratories for Virological Testing of National Institute of Health (Japan), who offered to Akhtar serological diagnostic services if samples be delivered from Karachi. But no sample was delivered to Tokyo for the next 2 years. Therefore, a new international study plan was thought out by Kono.
Collaborative Work between Japanese and Pakistani (1981 to 1986)
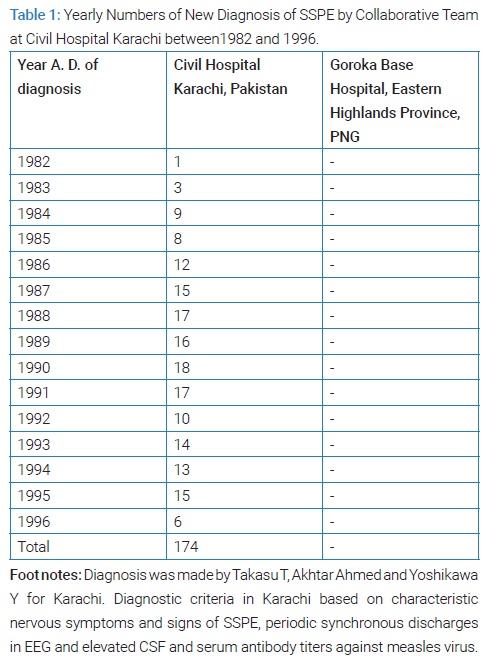
Called by him Takasu applied for Japan Ministry of Education and Culture (JMESC)’s Program for Overseas Scientific Surveys in 1980. Fortunately from 1981 to 1993 fiscal Takasu had an opportunity to lead a team organized multi-disciplinarily, inter-institutionally for the clinical, epidemiological, serological, epizootiological, entomological and virological studies aimed at the encephalitides occurring in Karachi, Pakistan, with particular attention to the 3 in question; the chronic encephalitis, perhaps SSPE, the acute encephalitis, perhaps Japanese encephalitis (JE), and the acute radiculo-myelitis associated with hemorrhagic conjunctivitis by Entero 70 virus in Pakistan. Mobilized Japanese researchers during 1982 to 1997 numbered 27 from 18 institutions and mobilized Pakistani researchers 9 from 4 institutions [58]. Takasu was funded a total of 87,300 thousands Japanese Yens including from Japanese Ministries of Education, Science and Culture and of Health and Welfare and Nihon University. During the 16 years 1981 to 1996, 26 Japanese visited Karachi in 104 times, and 10 Pakistani visited Japan in 11 times.
Actual work
During the period of time, for enabling the serological diagnosis of SSPE, JE, WNE (West Nile encephalitis) and Entero70-caused infections as well as the isolation and sequence analysis of viruses, the team members carried frozen serum, CSF and mosquito samples with dry ice with them back to Japan. In those days dry ice was unavailable in urban Karachi so the team must drive far to Karachi Airport where it was supplied. For virological studies the samples must be kept under minus 60 degrees, but suitable ultra low temperature freezer was not on the market in Karachi, so that the team had to carry the samples with themselves to store them in Aga Khan University Biochemistry Department with kind permission and cooperation by Professor and Director M. Anwar Waqar. For the purpose of home survey by the social worker in collaboration Takasu purchased a motor cycle in town for his free use.
Results of Collaboration in Karachi
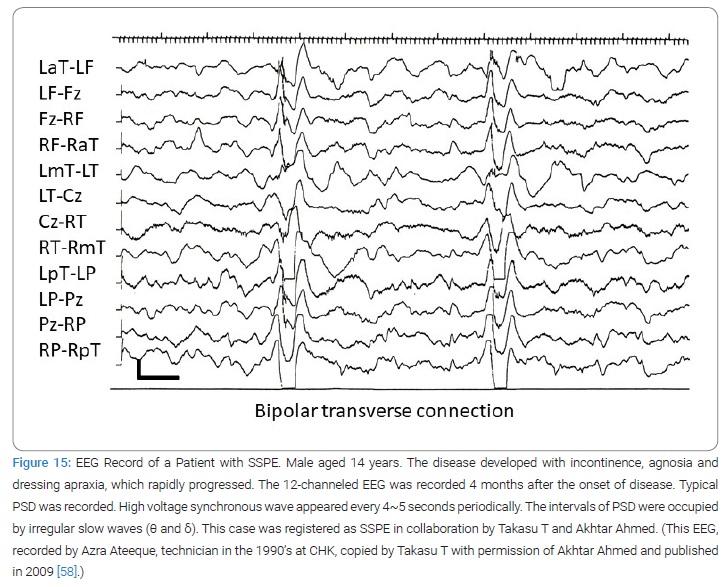
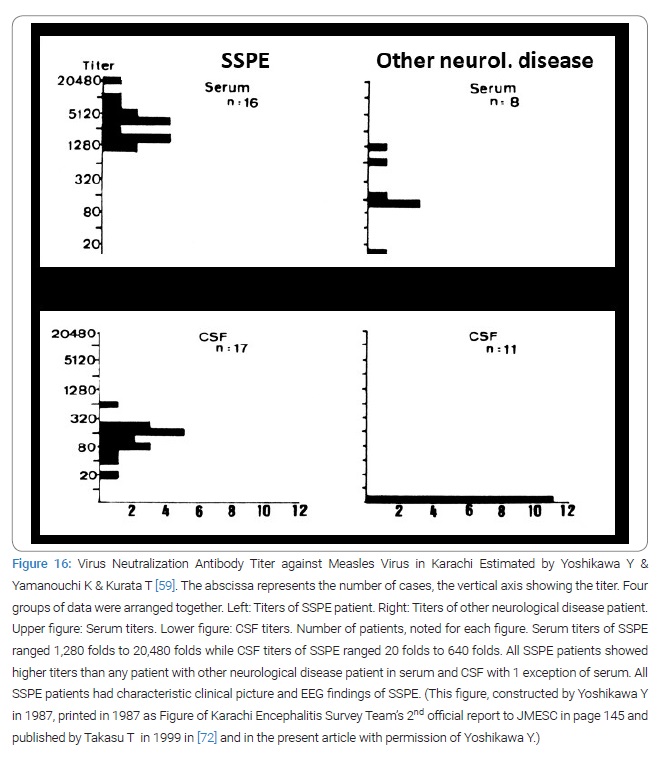
Thus from 1981 to 1997 Takasu and Akhtar diagnosed 174 SSPE at Civil Hospital Karachi (Table 1) (Figure 15 and 16) [59]. The diagnosis criteria of SSPE in Karachi was firstly the clinical set of mental and/or physical deterioration that appeared subacutely and observable myoclonic jerks and/or fits; secondly the periodic synchronous discharges recorded in EEG (Figure 15); thirdly elevated CSF and/or serum antibody titers to measles virus (Figure 16) [59]. The activities by the virologists, the entomologists, the epidemiologists and the veterinarians of the team were only minimally described in this paper to fit to the title of this paper. The published number of original paper was 17.
Four books in English were officially reported by Karachi Encephalitis Survey Team to Japan MESC which was distributed widely to the collaborators and persons concerned in Karachi. They were: 1st report. A Neuro-Viro-Patho-Epidemio-Entomological Survey. 1985; p. 1–105. 2nd report. A Neuro-Viro-Patho-Epidemio-Entomological Survey. 1987; p. 1–304. 3rd report. A Clinico-Epidemio-Viro-Sero-Immunogenetico-Entomo-Epizootiological Study. 1991; p. 1–257. 4th report: A Clinico-Epidemio-Viro-Sero-Immuno-Entomological Study. 1994; p. 1–240.
The whole aspect of the collaborative studies was summarized in the invited article by Japan Society for Promotion of Science [57]. The principal investigators were 13; the 2 neurologists Toshiaki Takasu and Akhtar Ahmed; the 4 virologists Masami Sugamata, Akira Igarashi, Yasuhiro Yoshikawa and Katsuhiro Komase; the 3 pediatricians: Shin Isomura, D. S . Akram and Mubina Agboatwalla; the 1 immunologist Keiji Terao; the 1 entomologist Kiyoshi Kamimura; the 2 epidemiologists Kiyotaro Kondo and Kimihiro Nakae; the 1 veterinarian virologist Ikuo Takshima. The Pakistani main collaborators were Altaf Ahmed, Yasmeen Akbani and M. Anwar Waqar.
Summary of Study Result in Karachi
Epidemiology
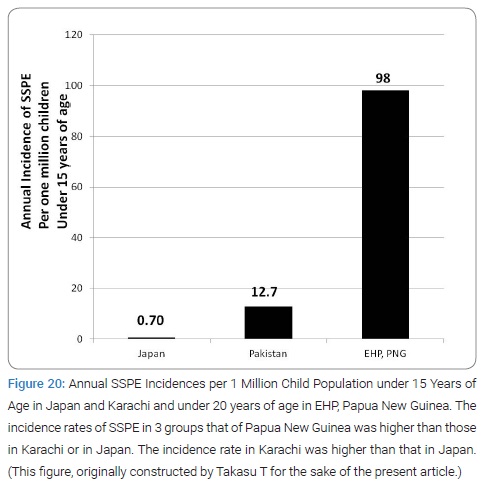
Annual Incidence of subacute sclerosing pan-encephalitis: The inferred annual incidence of SSPE in Karachi in 1974 to 1985 (6.35 per 106 population) was obtained from the formula: [the prevalence of SSPE in Karachi (1.25 per 10 thousand population)]/[the average survival of SSPE in year], where the prevalence of SSPE = [the relative number of recorded cases of SSPE to MND (motor neuron disease) at CHK between 1974 and 1985] х [ the universal average prevalence of MND for the 20 areas in the world (3.7 per 106 population) [60].The inferred annual SSPE incidence in Karachi per 106 child population under 15 years of age was roughly 12.7 since the population rate under 15 years was 43.8% (for 1972) and 44.4% (for 1978) [61–63]. These results were the 1st estimated value ever in whole Pakistan. The incidence value of SSPE in Karachi was roughly 10 times higher than in Japan or in USA [60,61].
Ratio of late measles in subacute sclerosing pan-encephalitis: Late measles (measles contacted at ≥ 2 years of age) occupied 65% of all measles histories of SSPE patients for 1983 to 1988 in Karachi, that was in striking contrast to 29% in whole Japan for 1966 to 1985 [62].
Attack rate of subacute sclerosing pan-encephalitis from late measles sufferers: The attack rate of SSPE from 105 early measles (below 2 years), late measles at 2 years–4 years or late measles at 5 or above years sufferers in Karachi being 23, 17 or 45 while in Japan they were 4.8, 1.3 and 0.6, respectively [56,62,63].
Risk factor of subacute sclerosing pan-encephalitis in Karachi: The SSPE patients in Karachi had abnormalities at birth with higher significance as risk factor of SSPE than their siblings or playmates and early measles with lower significance as risk factor of SSPE than in Japan or Europe or USA [61]. The elevated ratio of late measles in SSPE in Karachi [62] also indicated where the risk factor of SSPE was in Karachi and the presence of Karachi-region specific risk factor of SSPE [58]. This was the 1st case-control study in developing countries, which is ongoing.
Mothers’ knowledge of measles: The mothers’ knowledge of measles in Karachi proved fairly accurate and the clinical efficacy of measles vaccine proved around 72%. The age at measles in sporadic cases in Karachi was low and that in epidemic cases covered higher range [56].
Neurology
Features of subacute sclerosing pan-encephalitis in Karachi: Firstly late measles sufferers occupying the majority [62]; secondly by the latent period of SSPE from measles to SSPE onset, especially in late measles sufferers, being shorter; thirdly by fever around the onset of SSPE in about 25%; fourthly by myoclonus around the onset of SSPE in 36%; fifthly by fulminate onset in 33%; sixthly high efficacy of inosiplex; seventhly high positivity rate of IgM antibody against measles virus (56%) [63]. All these were resulted from an analysis of the 121 cases of SSPE diagnosed so at CHK during 1982 to 1992 according to the same diagnosis criteria as used in Europe or USA or Japan, the features so different from those known in Europe or USA or Japan, therefore all new as knowledge.
Virology
Sequence analysis of H, F and N genes of wild measles virus strains in Karachi: Phylogenetic tree analysis revealed that the 2 measles viruses isolated from PBMC of 2 patients with acute measles in Karachi could be classified into the same and new lineage when compared with other strains of measles virus in the world [64].
Serum HI antibody to 8 kinds of virus in healthy human: The serum HI antibody positivity rate in population resided in Karachi consisting of healthy volunteers and outpatients without any neurologic disease proved to be 69% to JEV, 70% to WNV, 72% to Dengue virus, 15% to Tick-borne encephalitis virus, 90% to Rubella virus, 82% to Measles virus, 66% and 20% to Herpes simplex virus type 1 for IgG or IgM antibody, respectively; 68% to Enterovirus 70 [57,65]. This was the 1st study in Pakistan on the antibody positivity rates to as many as kinds of viruses estimated simultaneously.
Serum antibody positivity rate to JEV and WNV in cows and buffalo: The serum HI antibody positivity rate was 36% to JEV and 55% to WN in cows and 63% to JEV and 76% to WNV in buffalos. Some individuals had the serum NT antibody to WNV alone while no individual had the serum NT antibody to JEV alone [57]; done by Takashima I, Go T, Hashimoto N, Waquar MA and Ashfaq K. No other study reported on the matter.
Existence of JEV in Karachi: The existence of JEV in Karachi was strongly inferred basing on firstly the 9 cases of acute encephalitis with significantly by more than 4 folds higher HI antibody titers to JEV than to WNV [57], done by Sugamata M, Takasu T and Ishii K; secondly 28 cases among 39 (72%) with elevated HI antibody titers to JEV, WNV or both accompanied by higher serum NT antibody titers to JEV by 2 folds to 32 folds than to WNV [57], done by Ishii K; thirdly the demonstrated JEV genome sequence in cerebrospinal fluid in 1 out of 24 patients with acute encephalitis [66]. This was important from virological point of view.
Existence of WNV in Karachi: The existence of WNV in Karachi was demonstrated by firstly the 1 case with by more than 4 folds higher HI antibody titers to WNV than to JEV found out in 1985, done by Takasu T; secondly the 1 case with by more than 4 folds higher serum NT antibody titer to WNV than to JEV among the 39 cases with elevated serum HI antibody titers to either or both of WNV or JEV, done by Ishii K; thirdly the isolated WNV in 4 stocks of Culex tritaeniorhyncus captured in Karachi [53], done by Kamimura K and Igarachi K; fourthly the higher serum HI antibody positivity rate to WNV than to JEV among the cows and buffalos from which blood sample was taken in August 1988 [57], done by Takashima I, Go T, Hashimoto N, Waqua MA and Ashfaq K; fifthly the demonstrated WNV genome sequence in cerebrospinal fluid from the 8 cases out of 24 of acute encephalitis [66].
Measles virus mutation in Karachi: The Measles virus mutation in the body was indicated by the 1 case of SSPE in Karachi of which the major antigen of HA (hemo-agglutination) was preserved for 4 years but the epitope of HA neutralization reaction mutated [57]; done by Yoshikawa Y.
Entomology
Prevalence of mosquitoes in Karachi: Five species of mosquitoes were prevalent in Karachi; Culex tritaeniorhynchus distributed in whole Karachi in low concentration, frequent in summer though seen in winter, too, but in Tatta and Hyderabad in high concentration; Anopheres stephensi was rare; Anopheres subpictus frequent in summer and autumn; Culex pipiens quinquefasciatus, frequent through the year; Aedes aegypti appeared only in the rainy season of summer in urban areas [57,67], done by Kamimura K. Four stocks of WNV were isolated from Culex tritaeniorhynchus captured in Katachi in July 1986 [57,67], done by Igarashi A, Kamimura K.
Human genetics
HLA in Karachi: The HLA (human leucocyte antigen) pattern in the residents in Karachi was different from any of the patterns in Japanese, Caucasian or Negroes [57]; done by Takasu T.
Clinical immunology
Immunoglobulin in children in Karachi: The low level children in Karachi in socioeconomic condition had on average high immunoglobulin values containing markedly high values and the high level children had on average low immunoglobulin values [68].
Environmental medicine
Heavy metals in human in Karachi: The cupper, iron, manganese and zinc concentrations in hair of the residents in Karachi were largely within normal range, relatively high values in cupper and iron in some and high values in mercury in those of medical occupation and in part of the workers in chemical factories, and largely high in fishermen [57]; done by Doi R.
Conclusions of studies in Karachi: Afterall, the SSPE-like chronic encephalopathy in question proved to be mostly SSPE.
JE virus and WNE virus existed in Karachi. JE and WNE occurred in Karachi in 1992. The Immersion of Entero70 virus proved to 68% in the residents in Karachi [57].
All the above activities and achievements prior to 1994 were reported in essence comprehensibly by Takasu T in the invited paper by Japan Society for Promotion of Science [57].
Subacute Sclerosing Pan-Encephalitis in Goroka (1996 to 2001)
Beginning: As a partial extension of the team activity in Karachi, the new team led by Toshiaki Takasu was organized focusing on the SSPE in EHP, PNG; the activity continued for another 6 years from 1996 to 2001. Mobilized number of Japanese researchers was 12 from 4 institutions and that of PNG researchers 6 from 2 institutions. This turn was ignited by the reported higher incidence of SSPE than in Karachi in PNG that was noticed to Takasu T by Toshiki Nishimura, a collaborator in Karachi [64]. With deep interest in the age at measles resemblance or difference in both areas Takasu T wrote to the author [69] of the paper requesting him to collaborate with the Japanese team but he already back to Australia. Some while afterwards Puka I. Tem, Secretary of Health, PNG Government, wrote to Takasu T, welcoming Takasu T’s team in PNG.
Goroka in the 1990’s: It was a town in EHP of PNG, located in the highlands about 1,600 meters (5,200 feet) above the sea level with a population about 19,000 in 2000 AD. Goroka was the capital of EHP. Goroka town was contained in Goroka District, that was 1 of the 8 districts of EHP. In the beginning of the 1990’s in EHP. The crude birth rate was 34.0 per thousand, the infantile mortality 82 per thousand, and the literacy rate over 10 years of age was from 23.5% to 30.2% of the 5 Highlands Provinces [70]. In Goroka Town GBH was situated as the sole general hospital in EHP. PNGIMR was located neighboring to GBH.
Collaborative work between Japanese and Papua New Guinean: Introduced by Tem the new collaborative work on SSPE started in 1996 by the Japanese team and the staffs of PNGIMR in Goroka, EHP, (Director: Michael P. Alpers) and of GBH. In particular Charles S. Mgone, Vice-Director and Head of Molecular Genetics, Joyce M. Mgone, Pediatrician-in-chief, Peter Asuo, Superintendent of GBH and Pediatrician, and Jack Markus, Pediatrician. Twelve Japanese researchers from 4 institutions and 6 Papua New Guinean researchers from 2 institutions participated in this activity [71,72]. In addition the collaborators in particular Kazumi Natsuhara from Japan and Goni Merenge and Graham Tagindo in PNG assisted the team. Takasu T was funded a total of 25,500 thousands Japanese Yens from JMESC, JMHW, JMHL, JMFA and 1,000 thousands Japanese Yens from Nihon University and 500 thousands Japanese Yens from civilian. Every half a year US $2,000 was contributed to PNGIMR for 1996 to 2001. Thus the boys with twitching muscles in Goroka in 1998 motivated the team members to work for those children (Figure 17) [71]. Ten Japanese visited PNG in a total of 36 times and 1 Papua New Guinean visited Japan in 1 time.
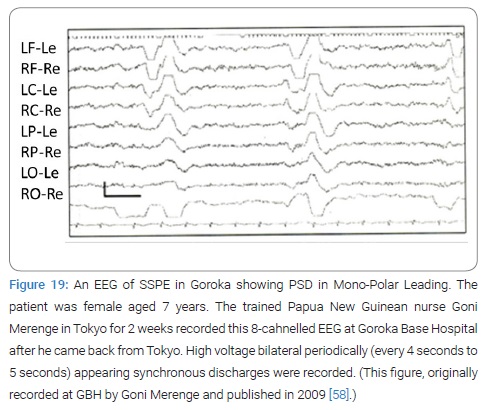
Actual work: On every visit Takasu T and Miki K carried with them frozen serum and CSF samples with dry ice or in liquid nitrogen back to Tokyo for antibody estimation of the samples in Tokyo because in those days there was no serologic diagnosis services for our study in Goroka or Port Moresby (Figure 18). Because no EEG was available in PNG in that time 1997, Takasu T purchased a 2-channel EEG instrument at first and next an 8-channel one in Japan which was sent to Goroka. Takasu T trained Papua New Guinean nurse Goni Merenge in Tokyo for EEG recording’s technique for 2 weeks at Nihon University Itabashi Hospital Neuro-Muscular Physiology Laboratories with assistance by the head technician. Later the trained nurse successfully recorded EEG back in Goroka (Figure 19).
Results of Collaboration in Goroka
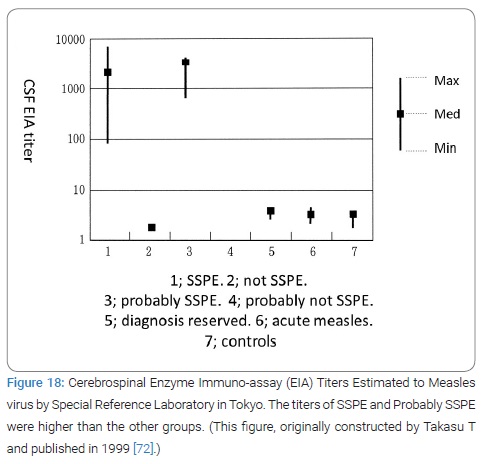
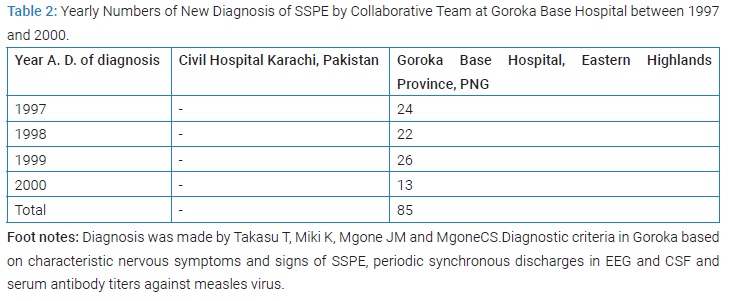
Takasu T and collaborators diagnosed 85 SSPE between 1997 and 2000 at GBH (Table 2) (Figure 18 and 19) [72]. The diagnosis criteria of SSPE in Goroka were essentially the same as Karachi that was described in detail in the Takasu T’s paper [73]. The serological study result in SSPE in Goroka was reported in 1999 (Figure 18 and 20) [72].
The 1st record of EEG in Papua New Guinea showed characteristic periodic synchronous discharges. Three original papers were published, 2 by Japanese and 1 by PNGIMR staff as 1st author, during the 6 years time [73–75]. The active investigators were 6: Toshiaki Takasu; Joyce M. Mgone, Kenji Miki, Postgraduate Student in Neurology, Nihon University School of Medicine; Charles S. Mgone; Michel P. Alpers; and Peter Asuo, Superintendent of GBH. The active collaborators were 4; Goni Merenge, nurse at GBH; Kazumi Natsuhara, postgraduate student in health, Tokyo University, Japan; Graham Tagindo, ex-school teacher and temporary taxi driver; and the Head technician of EEG laboratory at Nihon University Itabashi Hospital, Tokyo, Japan.
Another collaborative study between Takashi Ichiyama and Papua New Guinean researchers: Beside above, Ichiyama Takashi performed another independent collaborative study with P. Siba, then Director of PNGIMR, focusing on the cytokine dynamics and published 3 papers during the 6 years from 2002 to 2008 [58].
Summary and Conclusion of Study Result in Goroka
Incidence of subacute sclerosing pan-encephalitis in Eastern Highlands Province: Firstly, the team confirmed the continuing very high incidence of SSPE in EHP. The annual incidence of SSPE in the Eastern Highlands of Papua New Guinea was 98 per million child population below the age of 20 years for the 2 years from from 1997 to 1999, nearly 10 times higher than in Karachi and 100 times higher than in Japan or in USA, the highest ever reported by the year of its publication 2003 (Figure 20) [73].
Clinical features of SSPE in PNG: The majority of SSPE children there had contracted infantile measles [75].
Measles virus genome sequence in Goroka: The identified measles virus genome sequences in the clinical specimens from 2 SSPE patients’ PBMCs and 11 acute measles patients’ throat swabs were of genotype D3 with no similarity to any vaccine strain [74].
Conclusions of Part 2
- Human trust sometimes had obstacles surmounted.
- The result of the 2 studies on SSPE in 2 high incidence areas, Karachi and Goroka, is requiring risk factor analysis in those areas.
Ending remark of paper: As a person Takasu T often dreamed and as a scientist Takasu T did the same. Because of this inclination Takasu T often flied overseas between daily duties as clinician, educator or department director. Takasu T also is a slow-but-steady-type person. Time will be too short to change these inclinations. This is Takasu T’s actual footprint, right or wrong or good or bad.
Acknowledgment
The author thanks the late Former Chairman of SMON Research Commission Reisaku Kono for his understanding and acceptance of the author’s original work of GHT in SMON, his offering serological services for Akhtar Ahmed and his organizing the multi-disciplinary study team in Karachi.
The author thanks the editorial staffs for their invitation of Takasu T to this invited review article and their instructions and patience.
Conflict of Interest
The author declares no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Written permission was obtained from Ishiyaku Publishers, Inc. for reproducing the picture in Figure 13. For publicizing the picture of patient in Figure 14, the author strictly abided by the advice of Pakistani Professor Arsalan Ahmed, Ethics committee member of Shifa international Hospitals, Ltd., given to Takasu T in April 2022.
References
- Toyokura Y, Takasu T. Clinical Features of SMON. Jpn J Med Sci Biol. 1975;28 Suppl:87–99.
- Tsubaki T. Differential Diagnosis of SMON. Saishin Igaku. 1969;24(12):2465–2468.
- Tsubaki T, Homma Y, Hoshi M. Epidemiological Study on Clioquinol as the Cause of SMON. Jpn Med J. 1971;2448:29–34.
- Shimada Y, Toyokura Y, Matsuyama H, Kono R. About Subacute myelo-optic neuropathy. Record of table talk. Medicina. 1969;6:44–53.
- Toyokura Y, Tsukagoshi H, Igata A, Takasu T, Nakanishi T, Sugita H, et al. Studies on the clinical features, neuropathology and etiology of SMON. SMON Research Commission. Report number: 2 Clinical Sect. 1971;p.159–173.
- Toyokura Y. From SMON to clioquinol. Science i. e. Kagaku. 1972;42:532–543.
- Sobue I, Toyokura Y, Tamura Z, Ishii K, Shigematsu I, Omura I, et al. Recollection of the studies on SMON. Record of Table Talking; SMON Research Committee: Ando K. Report for fiscal. 1992;Suppl.1993;p.1–79.
- Takasu T. IV Sensory disturbance by drug “clinical case” SMON. In: Nakanishi A, Akiyoshi M. (eds.) Drugs and Sensory Disturbance. Tokyo: Soft Science. 1980.p.349–400.
- Takasaki H. Non-specific encephalomyelitis. In: Current view of internal medicine yearly supplement. Tokyo; Nakayama Book store. 1966.
- Takasaki H. SMON. Tokyo: Igaku Shoin Ltd. 1967.
- Takasaki H, Kanamaru M. SMON. Tokyo: Igaku Shoin Ltd. 1974.
- Takasu T, Igata A, Toyokura Y. The green hairy tongue observed in patients with SMON. J Clin Exp Med. 1970;72:539–540.
- Igata A, Takasu T, Toyokura Y. Green substance in the feces of SMON patients (Predictive Report). J Clin Exp Med. 1970;72(12):637–638.
- Kusui K. Outline of the joint SMON investigation by the clinical section. Jpn J Med Sci Biol. 1975;28 Suppl:57–62.
- Igata A, Hasebe M, Tsuji T. Green pigment of SMON patients-2 cases of green urine. Jpn Med J. 1970; 2421:25–28.
- Kosaka K, Shimada Y. Clinical study on SMON. [Presentation] SMON research commission 1st annual research report meeting for fiscal. Tokyo. Distributed print. 1970 June 30.
- Yoshioka M, Tamura Z. The actuality of green pigment of SMON patients. J Clin Exp Med. 1970;74:320–322.
- Ikeda Y. Heavy metal analysis in the urine and tongue fur of SMON patients. [Presentation] SMON research commission 1st annual research meeting for fiscal. Tokyo, Japan. Distributed print. 1970 June 30.
- Kozuka H, Tsunoda N, Igata A, Takasu T. Analysis of green substance in the tongue fur of SMON patients. J Clin Exp Med. 1970;75:372–373.
- Imanari T, Tamura Z. Detection of clioquinol from green tongue fur of SMON patients. J Clin Exp Med. 1970; 75:547–548.
- Ogawa K, Tsutsumi A, Motoi S. Autopsy cases of SMON in Okayama areas. SMON Research Commission. Report number: 4, Report of the Pathology Section for Fiscal 1970. 1971;18–48.
- Egashira Y. Report of the pathology section. b. result of the whole nation autopsy cases study of SMON and the histopathological diagnosis criteria of SMON. SMON Research Commission. Report number: 12 for Fiscal 1971. 1972;41-42.
- Takasu T, Igata A, Toyokura Y. The relationship of green hairy tongue and nervous symptoms in SMON patients to clioquinol. Jpn Med J. 1970; 2427:24–32.
- Tsubaki T, Homma Y, Hoshi M. Epidemiological study on clioquinol as the cause of SMON. Jpn Med J. 1971;2448:29–34.
- Yoshitake Y, Igata A. Study on the SMON occurring after abdominal surgery-its relationship to clioquinol prescription. J Clin Exp Med. 1970;74:598–599.
- Kono R. Introductory review of subacute myelo-optico-neuropathy (SMON) and its studies done by the SMON research commission. Jpn J Med Sci Biol. 1975;28 Suppl:1–21.
- Shigematsu I, Yanagawa H, Yamamoto S, Nakae K. Epedemiological approach to SMON (Subacute Myelo-Optico-Myelopathy). Jpn J Med Sci Biol. 1975;28 Suppl:23–33.
- Section of Pathology (Chairman: Egashira Yasuyuki), SMON research commission. A short history of autopsy study of SMON in Japan and principal lesions of the spinal cord in 113 autopsy cases. Jpn J Med Sci Biol.1975;28 Suppl:63–67.
- Shiraki H. The neuropathology of subacute myelo-optico-neuropathy “SMON”, in the humans. Jpn J Med Sci Biol. 1975;28 Suppl:101–164.
- Tateishi J, Otsuki S. Experimental reproduction of SMON in animals by prolonged administration of clioquinol: clinic-pathological findings. Jpn J Med Sci Biol. 1975;28 Suppl:165–186.
- Tateishi J, Kuroda S, Ikeda H, Otsuki S. Neurotoxicity of iodoxyquinoline: A further study on beagle dogs. Jpn J Med Sci Biol. 1975;28 Suppl:187–195.
- Toyokura Y, Takasu T, Matsuoka O. Experimental studies utilizing radio-nuclides of clioquinol as tracer In Vivo. Jpn J Med Sci Biol. 1975;28 Suppl:79–86.
- Tamura Z. Clinical Chemistry of Clioquinol. Jpn J Med Sci Biol. 1975;28 Suppl:69–77.
- Kono R. Virus and humans. 1st edn. Tamagawa selection 142. Tokyo: Tamagawa University Publication Department. 1981.
- Takasu T. II. The 100 years’ vicissitude of internal medicine. The drug-induced disease, centering around SMON- the progress from epidemic to elucidation of the cause. J Jpn Soc Int Med. 2002;91 (The 100th anniversary of the foundation of the Japanese society of internal medicine memorial issue):138–149.
- Takasu T. SMON- a model of the iatrogenic disease. In: Kuzuhara S, Tashiro K (chairman). <Symposium 7-1> Iatrogenic Diseases and Nervous System Disorders Due to Bio- or Chemical Poisons. Proceedings of the 44th general meeting of Japan society of neurology, 15-17 May 2003, Yokohama, Japan. Clin Neurol. 2003;43:.866–869.
- Takasu T. Elucidation of the cause of SMON originated from green hairy tongue. In: Konagaya M (ed.). Meeting of persons concerned in SMON in 2016. Suzuka, Aichi Prefecture, Japan. SMON research committee. 2017. p.138–149.
- Iwashita H. History and present status of SMON. IRYO. 2001;55:510–515.
- Inoue N. Green gift of nature. Recollection of SMON research. Fukuoka: Kaichosha Co. Ltd. 2011.
- Bareggi SR, Cornelli U. Clioquinol: review of its mechanisms of action and clinical uses in neurodegenerative disorders. CNS Neurosci Ther. 2012;18(1):41–46.
- Konagaya M. Sequelae of patients with SMON of onset in young age. In: Konagaya M (ed.). The 2016 meeting of persons concerned in SMON: proceedings of the meeting, 8 October 2016, Tokyo. Suzuka, Aichi Prefecture: SMON Research Committee; 2017. p.5–21.
- Kuru S. Fifty Years after the identification of the cause of SMON. Clin Neurol. 2021;61:109–114.
- Tamura Z. Lessons of iatrogenic disease SMON. Pharmacia. 2005;41:22–26.
- Miyoshi I, Muguruma M, Uchita Y, Hiraki K. Study of the experimental green tongue in SMON. SMON symposium-its current studies. Jpn Med J. 1971;29:12–13.
- Takasu T, Aoki Y, Toyokura Y, Matsuoka. Accumulation of labeled clioquinol in the superficial layer of mouse’s tongue-in relation to the green hairy tongue in SMON. J Clin Exp Med. 1973;84:81–82.
- Mori O, Ogawa T. A little hisotlogy, 10th ed. Tokyo-Kyoto: Kanehara Publishing Co.: 1952.
- Takasu T. Pathogenesis of the toxic myelo-optico-neuropathy with clioquinol (5-Chloro-7-Iodo-8-Hydroxy-Quinoline)-a macro-auto-radiographic and radio-chemical study. Clin Neurol. 1974;14:232–237.
- Yagi Y, Matsuda M, Yagi K. Production of lipid peroxide in sciatic nerve by clioquinol chelate with iron. J Clin Exp Med. 1976;96:639–640.
- Bragulla HH, Homberger DG. Structure and function of keratin proteins in simple, stratified, keratinized and cornified epithelia. J Anat. 2009;214:516–559.
- Yamashita S, Nakama T, Ueda M, Honda S, Kimura En, Konagaya M, et al. Tongue strength in patients with subacute myelo-optico-neuropathy. J Clin Neurosci. 2018;47:84–88.
- Manabe M, Lim HW, Winzer M, Loomis CA. Architectural organization of filiform papillae in normal and black hairy tongue epithelium: dissection of differentiation pathways in a complex human epithelium according to their patterns of keratin expression. Arch Dermatol. 1999;135:177–181.
- Brychtova V, Coates P, Hrabal V, Bordrup L, Fabian P, Voitesek B, et al. Keratin 36, a specific marker of tongue filiform papillae is down regulated in squamous cell carcinoma of the mobile tongue. Mol Clin Oncol. 2020;12:421–428.
- Lu CH, MacDonald-Willis C, Gray E, Pearce N, Petzold A, Norgren N, et al. Neurofilament light chain. Neurology. 2015;4:2247–2257.
- Ahmed A. Sub-acute sclerosing pan encephalitis a report of seven cases. J Pk Med Ass. 1980:249–251.
- Nakae K, Kondo K, Kamei S, Takasu T. Estimated mortality rate by sex, age and death causes in Karachi. J Pk Med Ass. 1986;36:174–176.
- Isomura S, Akhtar Ahmed, Akram DS, Agboatwalla M, Takasu T. Epidemiological studies of measles in Karachi, Pakistan-mothers’ knowledge, attitude and beliefs about measles and measles vaccine. Acta Paed Jpn. 1992;34:290–294.
- Takasu T. Subacute sclerosing panencephalitis and the Japanese encephalitis-like disease in Pakistan. Jpn Sci Monthly. 1994;47:48–57.
- Takasu T. Neuro-Infectious diseases and international cooperation-subacute sclerosing panencephalitis in Karachi, Pakistan, and Goroka, Papaua New Guinea. Neuroinfection. 2009;14:13–24.
- Yoahikawa Y, Yamanouchi K, Kurata T. Chapter 6. Serological studies of SSPE in Karachi as well as histological, virological examinations and virus isolation studies of brain biopsy materials. Jpn Min Edu Cul. 1987.
- Kondo K, Takasu T, Ahmed A. Neurological diseases in Karachi-elevated occurrence of subacute sclerosing panencephalitis. Neuroepidem. 1988;7(2):66–80.
- Kondo K. Slow Virus infection with conventional virus. Subacute sclerosing panencephalitis 3) Epidemiology. In: Yamanouchi K, Tateishi J, editors. Slow Virus Infection and Prion. Tokyo: Kindai Publishing Co.; 1995. p. 31–45.
- Takasu T, Kondo K, Ahmed A. Elevated ratio of late measles among subacute sclerosing pan-encephlitisin Karachi, Pakistan. Neuroepidm. 1992;11(4–6):282–287.
- Takasu T. Slow Virus infection with conventional virus. Subacute sclerosing panencephalitis 6) Features of SSPE in Karachi. In: Yamanouchi K, Tateishi J, editors. Slow virus infection and prion. Tokyo: Kindai Publishing Co.; 1995. p. 58–74.
- Nishimura T, Komase K, Terao K, Agboatwalla M, Akbani Y, Akram DS, et al. Sequence analysis of the H, F and N genes of wild measles virus strains isolated in Karachi, Pakistan. Nihon Univ J Med. 1997;39:323–336.
- Sugamata M, Ahmed A, Miura T, Takasu T, Kono R, Ogata T, et al. Sero-epidemiological study of infection with West Nile virus in Karachi, Pakistan, in 1983 and 1985. J Med Virol. 1988;26:243–247.
- Igarashi A, Tanaka M, Morita K, Takasu T, Ahmed A, et al. Detection of West Nile and Japanese encephalitis virus sequences in cerebrospinal fluid collected from acute encephalitis cases in Karachi, Pakistan. Microbiol Immunol. 1994;38:827–830.
- Kamimura K, Takasu T, Akhtar Ahmed. A survey of mosquitoes in Karachi area, J Pk Med Ass. 1986;36:182–187.
- Terao K, Shahid M, Kazmi SU, Akram DS, Agboatwalla M, Takasu T, et al. The immune function and measles virus infection in three different socioeconomic child populations in Karachi, Pakistan. Jpn J Med Sci Biol. 1994;47:87–99.
- Lucas KM, Sanders RC, Rongap A, Pina S, Alpers MP. Subacute sclerosing panencephalitis (SSPE) in Papua New Guinea: a high incidence in young children. Epidem Infect. 1992;108:547–553.
- Papua New Guinea dept. of health national health plan 1996–2000. Papua New Guinea government. Report number: 2,1996. p. 1–195.
- Takasu T, Kokubun Y, Nishimura T, Miki K, Kawanishi, Komase K, et al. Subacute Ssclerosing panencephalitis, measles and measles vaccination in Papua New Guinea-scientific investigation, collaborative research and studies, and non-governmental organization activities-(1). Nettai. 1998;31:251–258.
- Takasu T, Miki K, Komase K, Kawanishi R, Yoshikawa Y, Kokubun Y, et al. Subacute sclerosing panencephalitis, measles and measles vaccination in Papua New Guinea-scientific investigation, collaborative research and studies, and non-governmental organization activities-(2). Nettai. 1999;32:149–161.
- Takasu T, Mgone JM, Mgone CS, Miki K, Komase K, Namae H, et al. A continuing high incidence of subacute sclerosing panencephalitis (SSPE) in the Eastern Highlands of Papua New Guinea. Epidem Infect. 2003;131:887–898.
- Miki K, Komase K, Mgone CS, .Kawanishi R, Iijima M, Mgone JM, et al. Molecular analysis of measles virus genome derived from SSPE and acute measles in Papua New Guinea. J Med Virol. 2002;68:105–112.
- Mgone CS, Mgone JM, Takasu T, Miki K, Kawanishi R, Asuo PG, et al. Clinical presentation of subacute sclerosing panencephalitis in Papua New Guinea. Trop Med Intern Health. 2003;8:219–227.
Keywords
Green hairy tongue; SMON; Clioquinol; Lingual hyperkeratosis; SSPE; Karachi; Goroka
Cite this article
Takasu T. Green hairy tongue in subcute myelo-optico-neuropathy (SMON) and boys with twitching muscles in karachi and in goroka with subacute sclerosing pan-encephalitisas (SSPE): 2 clinical findings that led research and studies. Ann Neur Res Stud. 2022;1(1):1–23.
Copyright
© 2022 Toshiaki Takasu. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).






















