SFTSV Detection in Blood Sucking Arthropods and Vector Competence of Tick Species for SFTSV
* Fu R;
* Tao H;
Sun Y;
Huang X;
* Zheng W;
-
* Fu R: The Collaboration Unit for Field Epidemiology of State Key Laboratory for Infectious Disease Prevention and Control, Jiangxi Provincial key Laboratory of Animal-origin and Vector-borne Diseases, Nanchang Center for Disease Control and Prevention, Nanchang, China.
-
* Tao H: The Collaboration Unit for Field Epidemiology of State Key Laboratory for Infectious Disease Prevention and Control, Jiangxi Provincial key Laboratory of Animal-origin and Vector-borne Diseases, Nanchang Center for Disease Control and Prevention, Nanchang, China.
-
Sun Y: State Key Laboratory of Pathogen and Biosecurity, Beijing Institute of Microbiology and Epidemiology, Beijing, China.
-
Huang X: National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
-
* Zheng W: The Collaboration Unit for Field Epidemiology of State Key Laboratory for Infectious Disease Prevention and Control, Jiangxi Provincial key Laboratory of Animal-origin and Vector-borne Diseases, Nanchang Center for Disease Control and Prevention, Nanchang, China.
-
Jul 01, 2022 |
-
Volume: 1 |
-
Issue: 1 |
-
Views: 1097 |
-
Downloads: 1101 |
Abstract
There is an increasing trend for detection of Severe Fever with Thrombocytopenia Syndrome Virus (SFTSV) in ticks collected from human, livestock, wild animals, birds and the environment. This information facilitates risk evaluation for SFTSV transmission by ticks, however, the accumulated information also presents challenge: how detection of SFTSV in ticks should best be interpreted. To serve as vectors of SFTSV, the tick species must acquire the pathogen at the immature stages during feeding on an infected host, and then transmit transstadially to the subsequent stages. The newly molted ticks are also capable of passing the pathogen to their bitten hosts. This review explores the evidences for and against the tick species serves as vectors of SFTSV. Of 23 tick species, Haemaphysalis longicornis is highly effective vector under laboratory conditions and frequently detected SFTSV positive in nature. Due to low abundance in nature, Ixodes sinensis was rarely identified and detected negative in small-sized sample. However, the tick species has been experimentally confirmed as vectors of SFTSV. In striking contrast, two tick species including Ixodes persulcatus, Dermacentor silvarum and three species of mosquitoes like Culex pipiens pallens, Aedes aegypti and Anopheles sinensis have been analyzed so far, and confirmed against serving as vectors of SFTSV. The remaining 19 ticks were detected positive or negative when they were collected from animals and vegetation at developmental stages. Judged by these data, nothing can be concluded regarding vector competence of the tick species for SFTSV. Therefore, the coming effort should be required to demonstrate their vector competence under laboratory conditions.
Introduction
Severe Fever with Thrombocytopenia Syndrome (SFTS) was first recognized in rural areas of China, and now becomes an endemic emerging infectious disease in China, Japan, and South Korea. SFTS human cases are also subsequently reported in Vietnam and Thailand, indicating that SFTS has already spread from East Asia to South East Asia [1–4]. SFTS has been part of severe public health problems in China, South Korea, and Japan. In China, SFTS cases have been mainly found in the Eastern, Central, and North-Eastern regions. Between 2010 and 2017, a total of 6515 laboratory-confirmed cases and 413 deaths were reported in mainland China [5]. An annual increase has been presented in the number and notification rate of SFTS cases [6]. The national case-fatality rate is 7.3%, ranging from 6.3% to 30.0% (Liu et al., 2014) [1]. In Japan, 319 cases were reported between 2013 and 2017 and the cumulative case fatality rate was 17% [7]. In Korea, 595 patients were detected for SFTS infection from 2013 to 2017 and the average fatality was around 21% [8].
Due to serious threat to public health with special emphasis on people who live, work and entertain in the rural areas, for approximately 10 years, great research progress has been made in the various aspects of SFTS concerns including SFTS discovery, SFTS Virus (SFTSV) structure and biology, vector and host, SFTS symptom, laboratory findings, pathophysiology, epidemiology, diagnosis, prevention and treatment [1,6,9,10]. Herein, focus of the present review is on the latest developments in SFTSV detection in arthropods and the assessment of the ability of arthropods as vectors to transmit SFTSV.
SFTSV presence in blood sucking arthropods
SFTSV is a novel-typed phlebovirus of the family Bunyaviridae. Most phleboviruses have previously proved associated with arthropods. Toscana virus and Punta Toro virus have been isolated from sandflies. The nonpathogenic Uukuniemi virus is a tick-borne phlebovirus. Rift Valley fever virus is transmitted mainly by mosquitoes. Positivity of arthropods commonly found in SFTS patients’ home environment for the first time spurs various subsequent detections of SFTSV in blood sucking insects, mites and ticks [4]. Epidemiological studies conducted in China, South Korea and Japan in the 2010s indicated a linkage between SFTS and bites by ixodid ticks [4,10,11]. At the SFTSV discovery, 10 of 186 ticks (5.4%) of the species Haemaphysalis longicornis contained SFTSV RNA and the ticks were harvested from domestic animals in the areas where the patients lived. The RNA sequences of these viruses were very closely related to the SFTSV isolated in samples obtained from the patients [4].
H. longicornis is widely distributed in East Asia including China, Korea, Japan, and South Pacific region like Australia, the Pacific Islands, and New Zealand. It has been confirmed as the predominant tick species in most regions of China, Korea, and Japan, with species composition of at least 52.58% [12–14]. Therefore, the tick species has been intensively used to detect the SFTSV infections in endemic and non-endemic regions to evaluate the risk of the virus spread. According to incomplete statistics, from 2012 to 2022, there are at least 20 studies dealing with SFTSV detections in H. longicornis collected from vegetation, domestic animals and wild animals. In vegetation, viral RNA, typically at low levels, was detected in only a small proportion of the larvae studied (0.05% to 0.78%), with no infection in two studies [12,14–17]; 0.07% to 3.36% of the nymphs were positive for SFTSV with no infection in two studies [11–21]; 0.12% to 5.41% of the males were positive with no infection in four studies [12,14,15,17,18,20,22]; and 0.35% to 9.38% of the females were positive with no infection in five studies [12,14,15,17,19,22]. On domestic animals, 1.5% to 3.0% of ticks from cattle were positive with no infection in two studies; positivity of 5.28% to 11.11% of ticks from sheep were identified; SFTSV was also detected, with infection rates of 2.1% to 4.97% of ticks from dogs with no infection in one study; Zero infections were found among the ticks removed from pigs and chickens [12,23–26]. On wild animals, SFTSV has been detected in the larvae (prevalence of 0.78% to 0.83%), 0.54% to 7.05% of the nymphs, 0.70% to 8.77% of the males, and 0.41% to 3.76% of the females [8,12] (Table 1). It seems that higher infections occurred in H. longicornis ticks post their exposure to the hosts and ticks attached on wild animals had higher infection rates than on domestic animals.
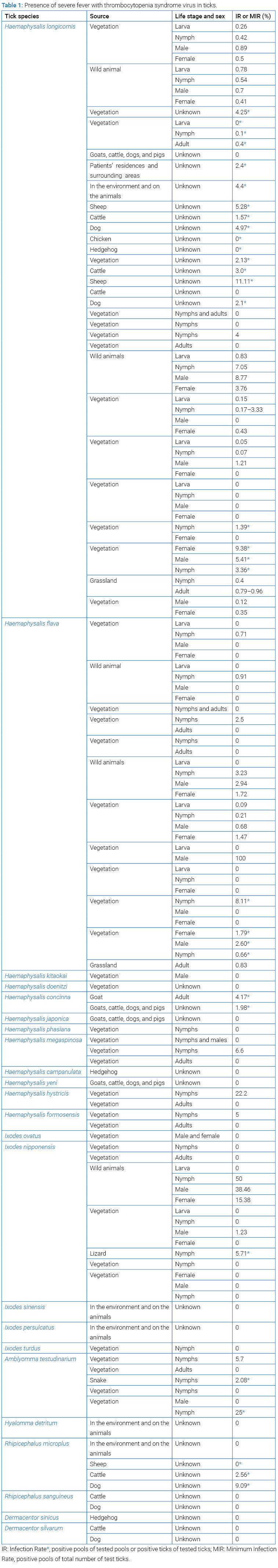
Haemaphysalis flava is subordinate only to H. longicornis in abundance in the non-endemic and endemic regions, and the second most used tick species for SFTSV detection. They have been frequently detected positive for the virus, whereas the infection rate was lower than that in H. longicornis. In vegetation, larvae were rarely found and had low chance to SFTSV infection, with rate of 0.09% in one study and no infection in other two studies [12,14,17,27]; nymphs were frequently identified and tested highly positive, and the positivities ranged from 0.21% to 3.23% as well as no infection in three studies [12,14,15,18]; males were easily observed but their counts were low, and less than 100 ticks, sometimes even less than 10 ticks, and the infection rates changed from 0.68% to 2.6% in two studies with one positive tick detected in one and five ticks in another two studies, respectively. The remaining two studies report no SFTSV infection in males [12,14,15,17–19]; like males, females also were the other stage commonly found and 1.47% to 1.70% of them were positive, while there were no infections in three studies though the results in the two studies were derived from small sample size [12,14,15,18,19]. On wild animals, nymphs were prone to SFTSV infection compared to larvae, females and males with the rates of 0.91% to 3.23%; in addition, ticks from medium-sized animals including deer, gorals, raccoon dogs, and wild boar have higher prevalence than those obtained from small mammals like hedgehogs, shrews and rodents [8,12] (Table 1).
Taken together, H. flava is commonly observed tick species in SFTSV endemic areas, and they are the second most frequently used ticks for virus detection. Generally, H. flava nymphs were more easily collected and highly positive for SFTSV presence compared to other developmental stages, moreover, ticks attached on medium-sized wild animals are prone to SFTSV infection versus those on small wild animals.
Besides H. longicornis and H. flava positive for SFTSV infection, SFTSV was also detected in 5.08% Haemaphysalis formosensis, 1.23% Ixodes nipponensis and 5.71% Amblyomma testudinarium collected from vegetation [11,14]. The virus was also detected in ticks removed from hosts, including 1.98% Haemaphysalis concinna from goats, cattle, dogs, and pigs, 4.17% H. concinna from goats, 5.41% Ixodes nipponensis from lizards, 2.08% A. testudinarium from snakes, 9.09% Rhipicephalus microplus from dogs, and 2.56% R. microplus from cattle [25,26,28,29]. Moreover, SFTSV RNA was found in lowly abundant tick species in nature, and they are Haemaphysalis hystricis and Haemaphysalis megaspinosa [11,12]. Other tick species were also employed for SFTSV detection, but they are negative, comprising Haemaphysalis kitaokai, Haemaphysalis doenitzi, Haemaphysalis japonica, Haemaphysalis phasiana, Haemaphysalis yeni, Haemaphysalis campanulata, Ixodes sinensis, Ixodes turdus, Hyalomma detritum, Rhipicephalus sanguineus, Dermacentor sinicus, and Dermacentor silvarum [13,14,20,23,25,26,30,31] (Table 1).
In addition to ticks, SFTSV infection has also been found in mites from in an endemic region of Jiangsu, China, with three positive pools in gamasid mite collected from Apodemus agrarius, and one positive pool in gamasid mite collected from goats. Chigger mites collected from A. agrarius were divided into five pools and all pools were also found to carry SFTSV [32]. However, viral RNA was not detected in any of 5900 mosquitoes collected from the SFTSV endemic areas [4].
Vector competence studies with ticks and mosquitoes
Vector competence is defined as “the ability of a vector to transmit a disease” and initially used in assessing the ability of Anopheles mosquito as a vector to transmit malaria [33]. It normally comprises the capacity of a vector to be infected, maintain and transmit an infectious agent. In the present study, vector competence was defined as three distinct processes: (1) Acquisition of SFTSV by uninfected ticks feeding on infectious experimental hosts, (2) Maintenance of SFTSV through the molt to the next life stage (transstadial passage), and (3) Transmission of SFTSV to naïve hosts during a subsequent blood meal [5,16,30,34,35]. A tick species can be considered a vector of SFTSV if all three processes have been experimentally demonstrated.
Haemaphysalis longicornis is a highly efficient vector for SFTSV
H. longicornis geographical distribution covers regions between 18° to 53° latitude in the northern hemisphere and 16° to 45° latitude in the southern hemisphere, and to date the tick species has been recorded in ten countries, including China, Japan, South Korea, the USA, Australia, New Zealand, Russia, India, New Caledonia, and Vanuatu. H.longicornis can feed on various animal hosts, such as livestock, wild animals and birds [36]. Live stock, wild animals and birds are susceptible to SFTSV infection. In nature, wild animals like shrews, hedgehogs, deer, and wild boars were investigated positive for SFTSV, with the rates ranging from 1.2% to 4.76% [8,12]. Compared to wild animals, livestock are usually highly positive, for instance, 73.7% goats and 59.1% cattle were detected for the SFTSV presence. SFTSV was also frequently detected in H. longicornis ticks collected from the animal hosts [8,12,23,24,26,31]. On the other hand, questing H. longicornis ticks had lower infection rate (0.6%) than feeding ones (0.7%) collected from animals living in the same environment, suggesting that animals contribute to SFTSV spread to ticks [12] (Table 1). In conclusion, animals, particularly domestic animals like cattle and goats, carry SFTSV in their blood and are a major food source for the ticks that spread SFTSV. Therefore, animals may act as amplifying and reservoir hosts of SFTSV. H. longicornis ticks can acquire SFTSV during their animal blood meal, and then maintain and transmit transstadially the virus to the subsequent stages, evidenced by common SFTSV RNA detection in the unfed ticks collected from vegetation. Human cases and SFTSV acquisition by goats resulting from bites of SFTSV-positive H. longicornis ticks also directly reflect the efficiency of the tick species as a vector of the virus [30,37,38].
The wave of vector competence studies in the lab and semi-field area was spurred by the description of SFTSV frequently from naturally infected human-biting H. longicornis ticks. In 2015, Jiao and coworkers used naïve goats in a SFTSV endemic area for mosquito and tick infestation. Viral RNA was detected from free-living and parasitic H. longicornis ticks rather than dominant Aedes albopictus and Culex pipiens mosquitoes and from goats after ticks’ infestation. Subsequently sero-conversion was observed in all members of the animal cohort. It indicates that H. longicornis, probably acts as vector for SFTS pathogen and highly effectively transmits the virus to naïve goats [30]. In the same year, Luo and the colleagues conducted transmission studies between developmental stages of H. longicornis ticks and between ticks and mice. The results showed that ticks fed on SFTSV-infected mice could acquire the virus and transstadially and transovarially transmit it to other developmental stages of ticks. Furthermore, SFTSV-infected ticks could transmit the virus to mice during feeding. The collective findings indicate ticks could serve as a vector and reservoir of SFTSV [16]. In 2018, Zhuang et al. 2018 [5], reported SFTSV dissemination in ovaries, hemolymph and salivary glands after the virus acquisition. Infected H. longicornis ticks could transmit SFTSV successfully in both transovarial and transstadial modes. In addition, naive BALB/C mice infested with experimentally infected adults, larvae, and nymphs all became positive for SFTSV presence. These data implicates that the H. longicornis tick is a competent vector to transmit this virus [5]. In 2022, Zhang and the comrades found that the SFTSV susceptibility of parthenogenetic H. longicornis females was comparable to that of bisexual females performed in previous three studies under laboratory or semi-field conditions. In this study, a higher proportion of parthenogenetic ticks were collected from migratory birds captured at an SFTSV-endemic area. It suggests that parthenogenetic H. longicornis ticks, probably transported by migratory birds, play a major role in the rapid spread of SFTSV [35]. Finally, as shown in four abovementioned studies, the evidence to date indicates that H. longicornis is a highly efficient vector for SFTSV regardless of tick population and animal hosts used in the transmission experiment.
Ixodes sinensis ticks are capable of transmitting SFTSV and act as the virus vector
I. sinensis is distributed within China, specifically speaking, the tick species is endemic in southern regions including Fujian, Jiangxi, Hunan, Yunnan, Zhejiang, Anhui, and Hubei [39]. The tick species can infest many vertebrates, including large, medium-sized and small mammals. Furthermore, large mammals comprise cattle, buffaloes and leopards. Medium-sized mammals infested by the tick species are sheep and hare. Like other Ixodes species, mice are the main hosts of the tick. I. sinensis has been reported to attack humans in the natural environment, but humans are not their specific hosts [39,40,41]. Despite no SFTSV infection found in I. sinensis in nature, the virus can be transmitted by the tick species under laboratory conditions. The transovarial transmission was seen in the I. sinensis ticks with a rate of 40%, which also have the ability to transmit SFTSV horizontally to uninfected mice at 7 days after feeding [34]. Therefore, we should pay more attention to the virus spread and its threat to public health where I. sinensis is dense and domestic animals like cattle carry SFTSV in the natural environment.
Ixodes persulcatus, Dermacentor silvarum ticks, and mosquitoes are not efficient vectors for SFTSV
To date, there have been reports of no SFTSV infection in I. persulcatus and D. silvarum ticks in nature [24]. The transovarial SFTSV transmission was also not seen in I. persulcatus and in D. silvarum ticks. Furthermore, the two tick species have no ability to transmit SFTSV horizontally to uninfected mice. In the transstadial transmission, I. persulcatus ticks were tested negative from larvae to adults, whereas the D. silvarum ticks were tested positive from larvae to nymphs. However, the mice bitten by SFTSV-infected D. silvarum nymphs were negative for SFTSV detection. Therefore, there is not enough evidence to support the transstadial transmission of SFTSV in I. persalcatus and D. silvarum ticks [34].
As we known, mosquitoes are probably the most common vectors of infectious diseases like malaria, Dengue fever and Japanese Encephalitis, and they are identified as major vectors for transmission of multiple Bunyaviridae viruses such as Rift Valley fever. However, viral RNA was not detected in large number of mosquitoes collected from the SFTSV endemic areas [4]. A further transmission study conducted in the lab show that three dominant mosquito species Culex pipiens pallens, Aedes aegypti and Anopheles sinensis fed with SFTSV-contaminated hamster blood can acquire the virus through blood feeding. However, a decreasing tendency of SFTSV titers was observed in all three mosquito species with the extension of feeding, indicating no viral replication detected in mosquitoes. The results demonstrate that mosquitoes are not vectors for transmission of SFTSV [42].
Tick species likely to serve as vectors of SFTSV but still lacking formal experimental demonstration
H. flava is perhaps the best example of a tick species which almost certainly is a vector of SFTSV but where experimental demonstration of vector competence is still lacking (due to logistical challenges of conducting laboratory studies with this tick). There is strong evidence from several field studies, including frequent SFTSV detection in larval, nymphal, female and male H. flava ticks, particularly nymphs collected by sweeping the vegetation, to indicate that transstadial transmission of SFTSV are maintained by the tick species in the natural environments. Other examples of tick species where field evidence partially supports vector competence by questing ticks positive for SFTSV presence include A. testudinarium, I. nipponensis, H. megaspinosa, H. hystricis, and H. formosensis. Some tick species that deserve mention as likely vectors of SFTSV are R. microplus and H. concinna, which are investigated positive when collected from the highly positive goats, cattle and dogs, and these animals are the reservoir of SFTSV and the primary hosts of the two tick species. There are 12 ticks species reported SFTSV negative and they consist of H. kitaokai, H. doenitzi, H. japonica, H. phasiana, H. yeni, H. campanulata, I. sinensis, I. turdus, H. detritum, R. sanguineus, D. sinicus, and D. silvarum. Perhaps, these tick species are less likely to be highly efficient vectors, which greatly increases the effort required to verify their vector competence, and it is overestimated to label them incapable of acting as vectors of SFTSV without the additional studies [43,44].
Conclusions and Future Directions
Extensive studies involving SFTSV detection in the field have produced numerous records of SFTSV acquisition not only in parasitic H. longicornis ticks but also the questing ones. Moreover, there is direct evidence showing the tick acting as a vector of SFTSV by human cases and SFTSV acquisition by goats resulting from bites of SFTSV-positive H. longicornis. In addition, the tick H. longicornis has been experimentally confirmed as a highly efficient vector of SFTSV, and can readily transmit the pathogen to mice and goat by bites. As noted in a study, I. sinensis was detected negative for SFTSV. However, the transovarial transmission was seen in the I. sinensis ticks and they have the ability to transmit SFTSV horizontally to uninfected mice under laboratory conditions. Further field studies on the natural infection in this tick species are intensively needed although its abundance is low in nature. The tick species have been previously reported to infest human, increasing the chance to spread SFTSV. Compared to I. sinensis, efforts have been more intensive for H. flava with natural infection detection for SFTSV confirmed for immature and mature stages collected from hosts and habitats. The experimental evidence of vector competence ofH. flava for the virus is still lacking and renewed studies to confirm the vector competence of the tick for SFTSV are merited. The collective evidences from the field and lab indicates that I. persulcatus and D. silvarum ticks and mosquitoes are unlikely to contribute to transmission of SFTSV. SFTS control and prevention should be focused on other tick species rather than I. persulcatus and D. silvarum ticks in the future. Questing ticks positive for SFTSV are highly suspected as potential vectors and they are comprised of H. formosensis, I. nipponensis and A. testudinarium. The experimental data on the vector role of these tick species are necessitated to evaluate. Based on current data, some tick species like H. concinna, and R. microplus fed on the hosts were detected positive for SFTSV infection. Judged by these data, nothing can be concluded regarding the capacity of infected ticks to transmit the virus while feeding. Therefore, the importance of documenting the infection status of the ticks having fed on naïve hosts cannot be overemphasized. The coming effort should be required to demonstrate their vector competence. No infection detection demonstrates that SFTSV acquisition is likely ineffective for some tick species like H. kitaokai, H. detritum, R. sanguineus, and D. silvarum. Although none of these tick species are likely to be highly efficient vectors, which greatly increases the effort required to demonstrate their vector competence, it is overstated to label them incapable of serving as vectors of SFTSV without the benefit of additional studies.
Ticks acting as vectors should create friendly environments for the virus binding and propagation, including lowering cell intrinsic antiviral immunity, modulating the peritrophic matrix and the mucin layer, and modifying local symbiotic microorganisms. On the other hand, SFTSV needs to encode components to aid in penetration of epithelial cells, entry into hemolymph, and migration to salivary gland for contributing to ticks being efficient vectors for the virus. Future researches to better understand the mechanisms resulting in some ticks being permissive to infection with SFTSV are necessary and the identification of some key components of ticks as a vector facilitate more easily and rapidly evaluating whether the tick species is a vector of the virus or not than before.
Acknowledgments
This study was supported financially by Jiangxi Provincial Department of Science and Technology (Grant Number 20201BBG71010, 20192BBHL80013).
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Liu Q, He B, Huang SY, Wei F, Zhu XQ. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis. 2014;14(8):763–772.
- Daengnoi C, Ongkittikul S, Watanawong R, Rompho P. Severe fever with thrombocytopenia syndrome virus: the first case report in Thailand. Bangkok Medical Journal. 2020;16(2):204.
- Tran XC, Yun Y, Van An L, Kim SH, Thao NTP, Man PKC, et al. Endemic severe fever with thrombocytopenia syndrome, Vietnam. Emerg Infect Dis. 2019;25(5):1029–1031.
- Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364(16):1523–1532.
- Zhuang L, Sun Y, Cui XM, Tang F, Hu JG, Wang LY, et al. Transmission of severe fever with thrombocytopenia syndrome virus by Haemaphysalis longicornis ticks, China. Emerg Infect Dis. 2018;24(5):868–871.
- Zhan J, Wang Q, Cheng J, Hu B, Li J, Zhan F, et al. Current status of severe fever with thrombocytopenia syndrome in China. Virol Sin. 2017;32(1):51–62.
- Shimada T, Saijo M, OishiK. Epidemiology of severe fever with thrombocytopenia syndrome in Japan. Singapore: Springer. 2019;pp.103–107.
- Oh SS, Chae JB, Kang JG, Kim HC, Chong ST, Shin JH, et al. Detection of severe fever with thrombocytopenia syndrome virus from wild animals and Ixodidae ticks in the Republic of Korea. Vector Borne Zoonotic Dis. 2016;16(6):408–414.
- Li DX. Severe fever with thrombocytopenia syndrome: a newly discovered emerging infectious disease. Clin Microbiol Infect. 2015;21(7):614–620.
- Takahashi T. [Severe fever with thrombocytopenia syndrome (SFTS) and SFTS virus]. Uirusu. 2015;65(1):7–16.
- Sato Y, Mekata H, Sudaryatma PE, Kirino Y, Yamamoto S, Ando S, et al. Isolation of severe fever with thrombocytopenia syndrome virus from various tick species in area with human severe fever with thrombocytopenia syndrome cases. Vector Borne Zoonotic Dis. 2021;21(5):378–384.
- Li Z, Bao C, Hu J, Liu W, Wang X, Zhang L. Ecology of the tick-borne phlebovirus causing severe fever with thrombocytopenia syndrome in an endemic area of China. PLoS Negl Trop Dis. 2016;10(4):e0004574.
- Matsumoto N, Masuoka H, Hirayama K, Yamada A, Hotta K. Detection and phylogenetic analysis of phlebovirus, including severe fever with thrombocytopenia syndrome virus, in ticks collected from Tokyo, Japan. J Vet Med Sci. 2018;80(4):638–641.
- Yun SM, Lee YJ, Choi W, Kim HC, Chong ST, Chang KS, et al. Molecular detection of severe fever with thrombocytopenia syndrome and tick-borne encephalitis viruses in ixodid ticks collected from vegetation, Republic of Korea, 2014. Ticks Tick Borne Dis. 2016;7(5):970–978.
- Ham H, Jo S, Jang J, Choi S. No detection of severe fever with thrombocytopenia syndrome virus from ixodid ticks collected in Seoul. Korean J Parasitol. 2014;52(2):221–224.
- Luo LM, Zhao L, Wen HL, Zhang ZT, Liu JW, Fang LZ, et al. Haemaphysalis longicornis ticks as reservoir and vector of severe fever with thrombocytopenia syndrome virus in China. Emerg Infect Dis. 2015;21(10):1770–1776.
- Yun SM, Song BG, Choi W, Roh JY, Lee YJ, Park WI, et al. First isolation of severe fever with thrombocytopenia syndrome virus from Haemaphysalis longicornis ticks collected in severe fever with thrombocytopenia syndrome outbreak areas in the Republic of Korea. Vector Borne Zoonotic Dis. 2016;16(1):66–70.
- Chae JB, Kim TH, Jung JH, Park YJ, Park JH, Choi KS, et al. Prevalence of severe fever with thrombocytopenia syndrome virus among ticks surveyed at Mt. Gwanak, Korea. Korean J Vet Res. 2017;57(3):169–174.
- Jo YS, Kang JG, Chae JB, Cho YK, Shin JH, Jheong WH, et al. Prevalence of severe fever with thrombocytopenia syndrome virus in ticks collected from national parks in Korea. Vector Borne Zoonotic Dis. 2019;19(4):284–289.
- Kim JY, Jung M, Kho JW, Song H, Moon K, Kim YH, et al. Characterization of overwintering sites of Haemaphysalis longicornis (Acari: Ixodidae) and tick infection rate with severe fever with thrombocytopenia syndrome virus from eight provinces in South Korea. Ticks Tick Borne Dis. 2020;11(5);101490.
- Lee J, Moon K, Kim M, Lee WG, Lee HI, Park JK, et al. Seasonal distribution of Haemaphysalis longicornis (Acari: Ixodidae) and detection of SFTS virus in Gyeongbuk Province, Republic of Korea, 2018. Acta Trop. 2021;221:106012.
- Seo JW, Han SY, Sung SH, Jung EY, Kim JH, Lee SJ, et al. Survey on tick distribution and tick-borne pathogens in Daejeon and adjacent areas in South Korea. Ticks Tick Borne Dis. 2021;12(4):101711.
- Jiang XL, Wang XJ, Li JD, Ding SJ, Zhang QF, Qu J, et al. [Isolation,, identification and characterization of SFTS bunyavirus from ticks collected on the surface of domestic animals]. Bing Du Xue Bao. 2012;28(3):252–257.
- Liu H, Li Z, Wang Z, He B, Wang S, Wei F, et al. The first molecular evidence of severe fever with thrombocytopenia syndrome virus in ticks in Jilin, Northeastern China. Ticks Tick Borne Dis. 2016;7(6):1280–1283.
- Tian H, Yu P, Chowell G, Li S, Wei J, Tian H, et al. Severe fever with thrombocytopenia syndrome virus in humans, domesticated animals, ticks, and mosquitoes, Shaanxi Province, China. Am J Trop Med Hyg. 2017;96(6):1346–1349.
- Wang S, Li J, Niu G, Wang X, Ding S, Jiang X, et al. SFTS virus in ticks in an endemic area of China. Am J Trop Med Hyg. 2015;92(4):684–689.
- Han XH, Ma Y, Liu HY, Li D, Wang Y, Jiang FH, et al. Identification of severe fever with thrombocytopenia syndrome virus genotypes in patients and ticks in Liaoning Province, China. Parasit Vector. 2022:15(1):120.
- Meng K, Sun W, Cheng Z, Guo H, Liu J, Chai T. First detection of severe fever with thrombocytopenia syndrome virus in the tick species haemaphysalis concinna in shandong province, China. Parasitol Res. 2015:114(12);4703–4707.
- Suh JH, Kim HC, Yun SM, Lim JW, Kim JH, Chong ST, et al. Detection of sfts virus in ixodes nipponensis and amblyomma testudinarium (ixodida: ixodidae) collected from reptiles in the Republic of Korea. J Med Entomol. 2016;53(3):584–590.
- Jiao Y, Qi X, Liu D, Zeng X, Han Y, Guo X, et al. Experimental and natural infections of goats with severe fever with thrombocytopenia syndrome virus: evidence for ticks as viral vector. PLoS Negl Trop Dis. 2015;9(10):e0004092.
- Xing X, Guan X, Liu L, Zhan J, Jiang H, Liu L, et al. Natural transmission model for severe fever with thrombocytopenia syndrome bunyavirus in villages of hubei province, China. Medicine (Baltimore). 2016;95(4):e2533.
- Wang QK, Ge HM, Li ZF, Shan YF, Cui L, Wang YP. Vector research of severe fever with thrombocytopenia syndrome virus in gamasid mites and chigger mites. Chin J Vector Biol & Control. 2012;23:452–454.
- Sallum MAM, Conn JE, Bergo ES, Laporta GZ, Chaves LSM, Bickersmith SA, et al. Vector competence, vectorial capacity of Nyssorhynchus darlingi and the basic reproduction number of Plasmodium vivax in agricultural settlements in the Amazonian Region of Brazil. Malar J. 2019;18(1):117.
- Hu YY, Zhuang L, Liu K, Sun Y, Dai K, Zhang XA, et al. Role of three tick species in the maintenance and transmission of Severe fever with thrombocytopenia syndrome virus in humans, domesticated animals, ticks, and mosquitoes, Shaanxi Province, China. PLoS Negl Trop Dis. 2020;14(6):e0008368.
- Zhang X, Zhao C, Cheng C, Zhang G, Yu T, Lawrence K, et al. Rapid spread of severe fever with thrombocytopenia syndrome virus by parthenogenetic asian longhorned ticks. Emerg Infect Dis. 2022;28(2):363–372.
- Zhao L, Li J, Cui X, Jia N, Wei J, Xia L, et al. Distribution of Haemaphysalis longicornis and associated pathogens: analysis of pooled data from a China field survey and global published data. Lancet Planet Health. 2020;4(8):e320–e329.
- Tong Y, Wang Q, Fu Y, Li S, Zhang Z, Zhang Z, et al. Molecular identification of severe fever with thrombocytopenia syndrome viruses from tick and bitten patient in Southeast China. Virol J. 2020;17(1):122.
- Wang M, Zuo J, Hu K. Identification of severe fever with thrombocytopenia syndrome virus in ticks collected from patients. Int J Infect Dis. 2014;29:82–83.
- Zhang YK, Zhang XY, Liu JZ. Ticks (Acari: Ixodoidea) in China: Geographical distribution, host diversity, and specificity. Arch Insect Biochem Physiol. 2019;102(3):e21544.
- Zheng W, Chen H, Liu X, Guo X, Fu R. Severe tick infestation in a hare and potential risk for transmitting pathogens to humans. Korean J Parasitol. 2011;49(4):419–422.
- Zheng W, Xuan X, Fu R, Tao H, Xu R, Liu Y, et al. Preliminary investigation of ixodid ticks in Jiangxi Province of Eastern China. Exp Appl Acarol. 2019;77(1):93–104.
- Liang SY, Chu HL, Guo XL, Wang W, Chen HN, Zhang YF, et al. Experimental infections of mosquitoes with severe fever with thrombocytopenia syndrome virus. Infect Dis Poverty. 2017;6(1):78.
- Hajdusek O, Sima R, Ayllon N, Jalovecka M, Perner J, de la Fuente J, et al. Interaction of the tick immune system with transmitted pathogens. Front Cell Infect Microbiol. 2013;3:26.
- Ma E, Zhu Y, Liu Z, Wei T, Wang P, Cheng G. Interaction of Viruses with the Insect Intestine. Annu Rev Virol. 2021;8(1):115–131.
Keywords
SFTSV; Vector; Tick species; Haemaphysalis longicornis ; Ixodes sinensis
Cite this article
Fu R, Tao H, Sun Y, Huang X, Zheng W. SFTSV detection in blood sucking arthropods and vector competence of tick species for SFTSV. World J Public Health Epidemiol. 2022;1(1):1–9.
Copyright
© 2022 Zheng W. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).

