Abstract
Invasive spinal Aspergillosis presenting as vertebral osteomyelitis and spinal epidural aspergillus abscess is a rare manifestation of fungal infection that usually presents in immunocompromised patients but is very rare in an immunocompetent adult. There are no reports of such infection in an immunocompetent patient after spine surgery. Also, complication as propriospinal myoclonus presenting after a year of spinal trauma is even rarer. We report a case of a patient with invasive spinal aspergillosis and paraplegia after the posterior instrumentation and stabilization with pedicle screws for burst L1 fracture that developed propriospinal myoclonus as a late complication. The infection subsided with two subsequent debridement and oral Voriconazole for six months, though the neurological deficit didn’t recover. Invasive spinal aspergillosis should be suspected even in immunocompetent patients, and early recognition for aggressive surgical intervention and antifungal therapy is needed to prevent complications.
Introduction
Invasive fungal spondylodiscitis is a rare condition usually presenting as an opportunistic infection in immunocompromised patients, and rarely affecting the immunocompetent patients [1–3]. Due to the delay in diagnosis which subsequently leads to delayed management, cases land up with complications. One of the complications is paraplegia secondary to cord transection which is irreversible [4–6]. A very rare complication arising from the cord injury is known as propriospinal myoclonus [7–9]. We present a very rare case of invasive spinal aspergillosis in an immunocompetent patient after spine surgery, with delayed diagnosis leading to paraplegia secondary to cord transection and presenting with features of Propriospinal Myoclonus (PSM).
Case Report
45-year-old male patient injured his back after a fall from a height and was diagnosed with an L1 burst fracture with intact neurology. He underwent spine stabilization with posterior instrumentation in a regional hospital. The patient recovered well after the surgery and was discharged within a few days. Six months later, he developed a surgical site infection. Surgical debridement and implant removal were done in the same center where the initial surgery was done. The patient was ambulatory immediately after the debridement. The patient developed gradual lower limb weakness associated with back pain and swelling over the surgical site. MRI was done which showed features of spondylodiscitis at L1 and L2 levels. A provisional diagnosis of Tubercular spondylodiscitis was made and anti-tubercular drugs were started empirically, without any culture or histopathology reports. The patient’s neurological status deteriorated to ASIA an impairment scale. The infection was fulminant with multiple discharging sinuses at the back. He was then referred for further management.
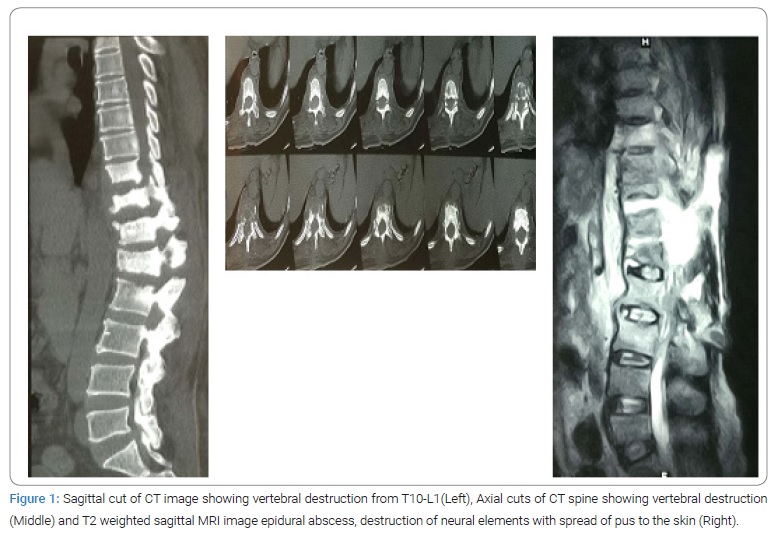
The patient had no other medical comorbid conditions that would suggest any immunocompromised state. All the blood reports were normal. X-ray of the lumbar spine showed features suggestive of spondylodiscitis of the T10-L1 vertebrae. CT scan showed lytic as well as sclerotic areas in the body and posterior elements of the T10-L1 vertebrae with endplate irregularities along with the reduced height (Figure 1). MRI was done which showed a decreased height of T10-L1 vertebrae with endplate erosions, posterior element destruction, paravertebral and epidural abscess, and abscess extending up to the epidermis posteriorly (Figure 1).
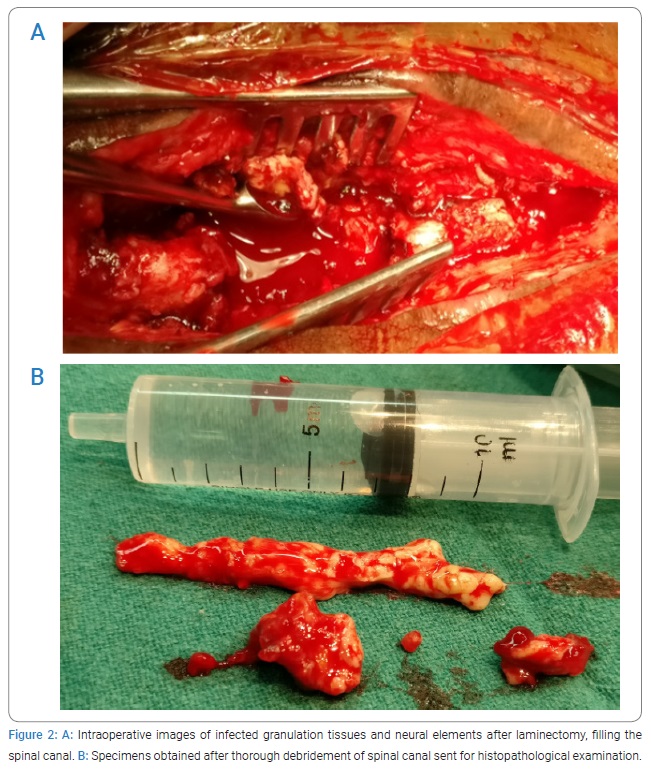
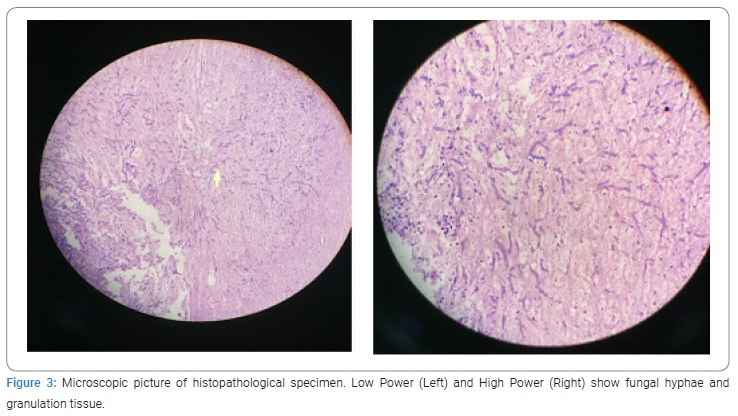
The patient was operated on with wide local debridement. The whole spinal canal was filled up with necrotic caseous material (Figures 2A and 2B). The infective process was so fulminant and widespread that demarcation between dura mater and neural elements was lost. There was a discontinuity in the spinal cord at the level of T10 to T12 and both the stumps were infiltrated by infective material, which could be easily curetted out. The histopathological report showed inflamed granulation tissue with aspergillosis (Figure 3).
Aspergillosis was shown in fungal culture. The antifungal drug, oral Voriconazole 200 mg twice a day was started. During three weeks of hospital stay, the clinical wound healing was better with the disappearance of the sinus tract and thus posterior stabilization with pedicle screws with additional anterior debridement and strut grafting was planned but the instrumentation was abandoned and wide surgical debridement was done because of the presence of fulminant infection intraoperatively. Oral Voriconazole 200 mg twice a day was used for maintenance therapy for six months. The patient returned after three months with increasing low back pain and swelling, for which an MRI was done which showed a recurrence of the disease. Since there was a recurrence of fulminant infection on oral Voriconazole, after consultation with infectious disease specialists, the use of Amphotericin was planned. The patient couldn’t afford liposomal Amphotericin; he was put on IV conventional Amphotericin B 0.5 mg/kg infused over 4 hrs for 7 days. After 7 days, the patient couldn’t tolerate the drug because of gastrointestinal side effects and electrolyte imbalance so he was put on oral Voriconazole 200 mg twice a day. After a few days, the patient developed intractable abdominal pain due to an involuntary spasm of abdominal muscles. Clinically it was diagnosed as Propriospinal Myoclonus (PSM) because of spinal cord injury. PSM is reported in many studies as functional (psychogenic) movement disorder. The pain responded only with a high dose of Clonazepam (2 mg thrice a day) for two weeks. The patient came back after a few days with intractable abdominal pain and vomiting. The hepatic physician diagnosed the patient with Voriconazole-induced hepatitis, and the drug was kept on hold.
A few weeks of supportive management was sufficient for liver functions to return to normal and Voriconazole was again restarted for another six months. The patient came for regular follow-up after 6 months. There were clinical signs of healing. X-ray and MRI revealed signs of healing. His neurological status however was the status quo.
Discussion
The usual causative organisms of spondylodiscitis are Mycobacterium tuberculosis or other bacterias [5]. Fungal spondylodiscitis is rare even in large series studies (6% to 9%) [10]. The incidence of fungal spondylodiscitis has increased in recent years, due to the increased number of immunocompromised patients and the rampant use of broad-spectrum antibiotics [6].
Common causative fungi are Candida and Aspergillus. Invasive aspergillosis usually involves the Sino-pulmonary tract, while the invasive spinal infection is uncommon [11]. Aspergillus osteomyelitis can occur due to contiguous spread or hematogenous route or direct implantation [12]. Nandeesh et al. [13] described fewer than 50 cases with aspergillus spondylodiscitis and almost all were immunocompromised. Sethi et al. [14] reported fifteen cases of Aspergillus spondylodiscitis in immunocompetent young adults. In 2016 Munjal S et al. [15] did a review of literature noting 22 cases of invasive aspergillosis of the thoracolumbar spine in immunocompetent patients, however, 6 of those cases had pulmonary aspergillosis and none of the cases were post-operative. However, we are not aware of any reports of aspergillus spondylodiscitis with the invasion of spinal neural elements in an immunocompetent patient after the spine operation. Our case might have acquired invasive spinal aspergillus infection during the operative procedure of implant removal and debridement. Aspergillus spondylodiscitis may present as back pain with the variable neurological deficit with or without fever, mimicking clinical features of pott’s spine [1,12]. Tuberculosis is considered to be the most common cause of spondylodiscitis in Nepal, clinic-radiological differentiation is a must. Pathogenesis of invasive fungal spinal osteomyelitis includes the erosion of body endplates with subsequent disc space narrowing and epidural abscess formation, thus mimicking the Pott’s spine [16]. This explains the diagnostic confusion and use of anti-tubercular drugs at the previous center. The histopathological examination and bacterial culture are often diagnostic [12,13].
Treatment advocated in aspergillus spondylodiscitis is wide debridement followed by posterior instrumentation with the addition of antifungals like Voriconazole, Itraconazole, Amphotericin B, Posaconazole for a total duration of 8 weeks to more than 6 months. Antifungal drugs have numerous side effects, so the treatment may be interrupted many times during the course [17]. The antifungal regimen of six months was interrupted twice, due to gastrointestinal and hepato-biliary side effects.
The patient also developed a very rare complication of spinal cord injury, PSM. The patient experienced repetitive, jerky movements of abdominal and thigh muscles. Diagnosis is clinical, supported by polymyography which shows a slow and progressive caudal propagation of muscle recruitment [7,8]. Clonazepam is the most effective drug to control symptoms of PSM and provided symptomatic relief in our patient as well [9].
Conclusion
When a clinician is confronted with a case of fulminant spondylodiscitis with multiple vertebrae involvement, extensive paravertebral and epidural abscess, and neurological deficit, invasive aspergillosis should be considered regardless of the immune status. Histopathological examination along with fungal stain and culture of a disco-vertebral specimen is fundamental to allow recognition and thus rapid initiation of a species targeted treatment. Aggressive surgical debridement and supportive antifungal therapy are necessary to prevent life-threatening invasive aspergillosis and irreversible spinal cord damage.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
Keywords
Aspergillosis; Immunocompetent; Post-operative complication; Propriospinal myoclonus; Voriconazole
Cite this article
Aspergillosis; Immunocompetent; Post-operative complication; Propriospinal myoclonus; Voriconazole
Copyright
© 2022 Sushil Paudel. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).



