Can Sarcopenia Also Affect the Gastrointestinal Tract of the Critically Ill Patient?
* Diogo Oliveira Toledo;
Castro MG;
de Pinho IC;
Prim C;
Waitzberg DL;
-
* Diogo Oliveira Toledo: Hospital Israelita Albert Einstein, São Paulo, Brazil; Brazilian Institute of Nutrition, Brasília, Brazil.
-
Castro MG: Hospital Israelita Albert Einstein, São Paulo, Brazil.
-
de Pinho IC: Division of Medicine, University College London, UK.
-
Prim C: Brazilian Institute of Nutrition, Brasília, Brazil.
-
Waitzberg DL: Faculty of Medicine, University of São Paulo, Brazil.
-
Oct 14, 2022 |
-
Volume: 1 |
-
Issue: 1 |
-
Views: 1306 |
-
Downloads: 951 |
Abstract
Sarcopenia is an important consequence of prolonged periods of hospitalization in the Intensive Care Unit (ICU), responsible for functional impairments and increased mortality. The Gastrointestinal Tract (GIT) dysfunction in critical patients may be related to structural or functional changes in smooth muscle of the GIT.
Introduction
Sarcopenia is defined as the progressive loss of muscle mass, strength, and function. It is primarily a consequence of aging and secondary to other causes, such as chronic conditions, malnutrition, inflammatory responses, as well as inactivity [1]. Sarcopenia is an important consequence of prolonged periods of hospitalization in the Intensive Care Unit (ICU), responsible for functional impairments and increased mortality. In addition, the muscular loss which patients suffer during more acute phases may endure for many years after the ICU discharge (Figure 1) [2–5]. The functional deficit developed during the hospitalization period can directly impact the patients’ quality of life, even in simple activities which were previously performed effortlessly.
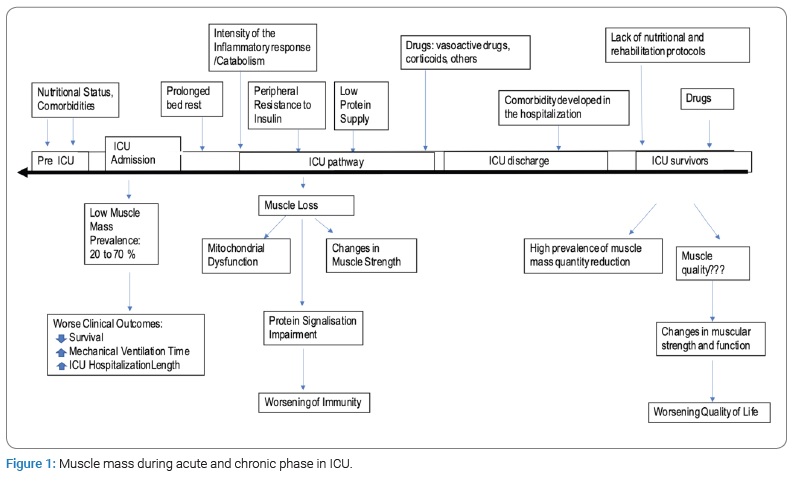
The inflammatory response intensity is a determining factor for the acute phase proteolysis degree. Muscles represent about 40% of body composition and are considered the essential tissue for movement [6,7]. It is important to mention that there are 3 types of muscle: skeletal, smooth and cardiac - and the inflammatory response caused by the acute disease are not selective to any particular type of them, being able to affect all three. On that note, it is a key to understand that the diaphragm is also a muscle and the main one when it comes to successfully weaning a patient from mechanical ventilation. In the same way, the Gastrointestinal Tract (GIT) is composed of smooth muscle and can also be compromised. In critical patients, skeletal muscle atrophy takes a prominent role in clinical evaluation due to the fact that it is more exposed, and it is easier to objectively measure its loss and function [7–9].
The present review highlights the importance of muscle wasting for critically ill patients and provides the rationale for the hypothesis that there is also a loss of smooth muscle during the accelerated muscle loss in the ICU, which may contribute to the dysfunction of the gastrointestinal tract.
Muscle Wasting and Functional Impairment
During severe inflammatory diseases, body protein from functional tissues is catabolized and may culminate in a significant loss of muscle mass [10]. The reduced muscle mass may impact in clinical outcome and leads to impaired functional ability, nosocomial infection, and wound infection [11]. This may induce a ‘vicious cycle’ of more complications that would intensify medical therapy, which may again result in increased morbidity and mortality [12–14].
Functional disability may be for long term and never fully return to normal levels. Importantly, it should be noted that muscular weakness will impact not only on individuals, but also on health care systems and global costs [12,13].
In the context of loss of muscle mass, it is known that there is also loss of diaphragmatic musculature. Ventilator‐induced diaphragmatic dysfunction can be noted in ICU patients after prolonged controlled mechanical ventilation and is determined by a rapid loss in force‐generating diaphragmatic capacity that may also affect additional respiratory muscles and often presents as failure to wean from mechanical ventilation despite sustained clinical efforts [13,15].
So, would it make sense to imagine that during exacerbated catabolism there would be a loss not only of skeletal muscle but also of smooth muscle?
GIT Dysfunction in the ICU
The GIT is almost entirely composed of smooth muscle. Thus, neurotransmitters activate the muscle fibers within range, and the excitation wave is conducted to all cells and bundles within the gap junction, thereby creating a morphological and functional syncytium [16,17].
During the acute inflammatory response, important morphological and functional changes may occur in the GIT. There are several causes for this, ranging from the intensity of the inflammatory response, the use of sedatives, analgesics, and neuromuscular blockers to hyperglycemia. Objectively quantifying and categorizing GTI dysfunction is a challenge in the ICU. This disfunction is relatively common and prevalent in patients with severe conditions. Changes such as slow gastric emptying, abdominal distension, abnormal motility patterns, and intestinal barrier vulnerability are observed in critical patients during the acute phase [16,17].
Changes in the GIT are markers for the extent and severity of the patient’s condition in the ICU. At the same time, the evolution of these pathophysiological changes is intrinsically associated with an inadequate progression of enteral nutritional therapy, energy and protein deficits, which leads to muscle mass loss and aggravated nutritional status [18,19]. If this cycle carries on uninterrupted, it may perpetuate the dysfunction of the GIT and consequently lead to adverse outcomes (Figure 2).
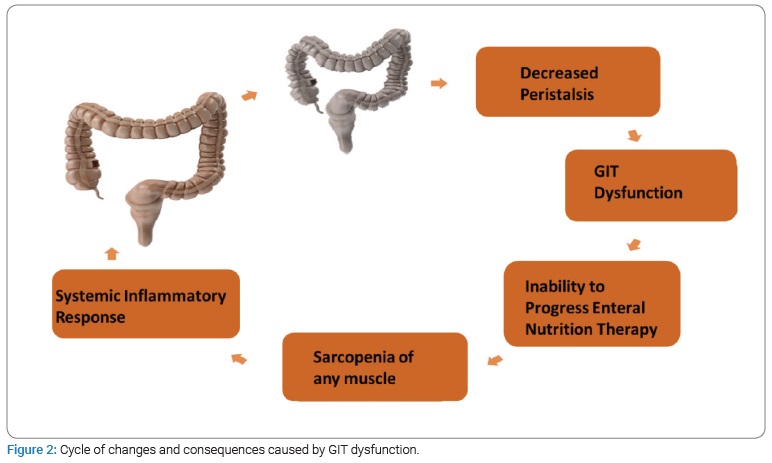
When it comes to clinical practice, the lack of an objective and standardized definition of what gastrointestinal dysfunction is, represents a major challenge. Consequently, this particular dysfunction is often recognized in a fairly intuitive way, based on each physician’s individual clinical experience. Furthermore, the lack of a specific marker (biological marker) to evaluate the viability of the GIT, especially after the adversities mentioned above, hampers the decision making of the physicians in the ICU, particularly when it comes to enteral nutrition.
Smooth Muscle Alterations during the Acute Inflammatory Response
Studies that demonstrate muscle mass reduction in critically ill patients use evaluation methods as computed tomography scan of L3, bioimpedance and ultrasound [9–21]. However, these studies evaluated skeletal muscle and not smooth muscle. Cardiac muscle has been implicated in the cachexia disease process, with pronounced atrophy and dysfunction in patients with pancreatic, lung, or colorectal cancer [21,22].
Weijs et al. [11] postulated in their review about protein in ICU that during starvation, the essential muscle mass of vital organs will also be reduced and, in combination with underused peripheral skeletal muscle, results in muscle weakness and functional disability.
Patejdl et al. [23] published an ex-vivo study aiming to evaluate the behavior and strength of smooth muscles during the inflammatory response. The authors used smooth muscle biopsies from patients who underwent elective GIT surgery. The biopsy sample was taken from a portion of non-compromised intestine (by tumor invasion or inflammation). After the biopsies, the samples were randomized into two groups: in one group, ICU patients’ serum were injected into the samples on days 3 and 10 and in the other group, serum from healthy patients were injected. The central idea was to use serum from patients during their acute inflammatory response, containing interleukins and inflammatory mediators from two different phases in the ICU. After in vitro preparation, these samples were compared to assess contraction, strength, and the synaptic transmission of smooth muscle. The result indicated worsening of contraction and strength in the subgroup of samples that were injected with serum from critically ill patients. In support of the hypothesis of smooth muscle loss during muscle wasting, a recent study identified symptoms of early satiety, diarrhea, and constipation in cachectic cancer patients that could be secondary to disturbed Gastrointestinal (GI) motility, a process intricately linked to proper intestinal smooth muscle function [24]. Moreover, several studies have described GI-motility disorders such as gastroparesis in patients with malignancies that are frequently accompanied by cachexia [25,26].
A recent study evaluated pancreatic cancer patients with and without sarcopenia. It was found that in sarcopenic patients, cancer cachexia affects also the intestinal smooth musculature together with skeletal and cardiac muscle wasting. In addition, both contractile function of smooth muscle cells and regulation of their contractile functionality could be compromised. The authors postulated that sarcopenic patients, due to the compromised gut function, could have significant impairment for their nutrient intake, transport, absorption, and faecal output and thus contribute to the development and progression of cachexia [27].
In the scientific literature, the major challenge is to evaluate, through imaging scans, the smooth muscle and its changes, such as its quantity and quality during the critical phase.
Increased muscle echodensity has been associated with muscle alterations in quality and function [28]. Moreover, gastric antrum echodensity has been proposed as a potential tool to evaluate the severity of acute gastrointestinal injury in critically ill patients undergoing mechanical ventilation [29].
How to rehabilitate smooth muscle composing the GIT?
The latest version of the European consensus of sarcopenia, accepted by the main specialized societies, advocates for new diagnostic criteria for sarcopenia [27,30,31]. Previously, strength was one of the main elements to evaluate muscle function (or quality). Now, strength takes on a key role in the diagnosis of sarcopenia, being used as a main parameter for the initiation of treatment. On the other hand, it is suggested that diminished amount, (or quality) of muscle mass should be used as a confirmatory criterion for the formal diagnosis of the disease. Thus, sarcopenia is said to be probable, when low muscular strength is identified, and it is confirmed in the presence of associated low muscle mass.
The strategies adopted for the treatment of sarcopenia are based on both protein supply and motor rehabilitation through resistance training [32,33]. However, these strategies were well established for skeletal muscle rehabilitation. Considering that the function of smooth muscles is motor propulsion and movement (almost like any skeletal muscle) it is logical to consider that its rehabilitation should follow the same principles. For that reason, offering an adequate amount of protein is essential. Nevertheless, the discrepancies between the universal recommendations for protein supply in critically ill patients (1.5 g/kg/day) and what most patients receive (0.8 g/kg/day to 1.0 g/kg/day, in the best scenarios) is worrisome [34]. The main nutritional therapy guidelines for critically ill patients recommend 1.2 g/kg/day to 2 g/kg/day. The FEED trial showed that the subgroup of ICU patients who received a higher protein supply (1.5 g/kg/day), was associated with improved muscle mass and strength, as well as improved nitrogen balance, when compared to the subgroup who received a lower supply of protein (1 g/kg/day) [35–37].
Protein quality should also be considered when choosing enteral diet formulae. Diets containing whey protein stimulate protein synthesis more efficiently than the ones with casein [38]. Furthermore, protein blends with balanced protein sources (containing whey protein) compared with predominantly casein-based formulations and appears to promote faster gastric emptying and less gastric accumulation. This effect seems to be more physiologically appropriate and clinically strategic [39].
Another important strategy in rehabilitating the smooth muscle of the GIT is restoring or improving peristalsis/motility. Studies show that early initiation of enteral/oral nutrition with intact (polymeric) formulae in critical and/or postoperative scenarios stimulates intestinal motility [40,41]. Moreover, formulations containing both soluble and insoluble fibers can be considered, if the hemodynamic condition and tissue perfusion are adequate [35]. The use of soluble and insoluble fibers is another strategy that contributes both to the management of changes in intestinal motility (diarrhea and constipation) and to the immunological strengthening of the severely ill patient [42–44]. Another study, using an experimental model demonstrated that the group that received fiber as a part of their nutritional strategy had greater power and strength in smooth muscle contraction, compared to the subgroup that did not receive any fiber [45].
Conclusion
GIT dysfunction in critically ill patients is a marker of condition severity and it is associated with negative outcomes. However, quantifying and classifying this dysfunction remains a challenge, considering the lack of uniformity of the validated tools and scores. Some of the unanswered questions about GIT dysfunction in critical patients may be related to structural or functional changes in smooth muscle of the GIT. An additional challenge lies in the methods of identification and diagnosis of smooth muscle abnormalities. So far, only ex vivo models have confirmed functional changes during the inflammatory response. The treatment and rehabilitation strategies for smooth muscle appear to and should be based on the same principles as the rehabilitation of skeletal muscles: adequate quantity and quality protein supply and mechanical stimulation of the muscles. Unfortunately, most studies showed that ICU patients rarely reach the protein goal, and very few studies evaluate and compared the quality of different protein sources. The early stimulation of GIT motility, through supplying food to the GIT should also be a strategy to rehabilitate smooth muscle. Another way to restore GIT motility during the inflammatory response is by offering fibers in the diet, however, this is contraindicated in some critical ill patients, and hence, it must be done cautiously. It is necessary to shed light on this issue, and to discuss alternative and simpler ways to identify smooth musculature during criticality, as well as strategies to rehabilitate this muscle type.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(4):16–31.
- Batt J, Santos CC, Cameron JI, Herridge MS. Intensive care unit-acquired weakness: clinical phenotypes and molecular mechanisms. Am J Respir Crit Care Med. 2013;187(3):238–246.
- Wang C, Bai L. Sarcopenia in the elderly: basic and clinical issues. Geriatr Gerontol Int. 2012;12(3):388–396.
- Chan KS, Mourtzakis M, Friedman LA, Dinglas VD, Hough CL, Ely EW, et al. Evaluating muscle mass in survivors of acute respiratory distress syndrome: a 1-year multicenter longitudinal study. Crit Care Med. 2018;46(8):1238–1246.
- Parry SM, Chapple LS, Mourtzakis M. Exploring the potential effectiveness of combining optimal nutrition with electrical stimulation to maintain muscle health in critical illness: a narrative review. Nutr Clin Pract. 2018;33(6):772-789.
- Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304.
- Toledo DO, Giorelli G, Castro MG. The critically ill patient: an athlete who never rest. World J Adv Healthc Res. 2019;3(2):69–72.
- Wischmeyer PE, Puthucheary Z, Millán IS, Butz D, Grocott MPW. Muscle mass and physical recovery in ICU: innovations for targeting of nutrition and exercise. Curr Opin Crit Care. 2017;23(4):269–278.
- Toledo DO, Carvalho AM, Oliveira AMRR, Toloi JM, Silva AC, Farah JFDM, et al. The use of computed tomography images as a prognostic marker in critically ill cancer patients. Clin Nutr ESPEN. 2018;25:114–120.
- Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–1600.
- Weijs PJM, Cynober L, DeLegge M, Kreymann G, Wernerman J, Wolfe RR. Proteins and amino acids are fundamental to optimal nutrition support in critically ill patients. Crit Care. 2014;18(6):591.
- Jolley SE, Bunnell AE, Hough CL. ICU‐acquired weakness. Chest. 2016;150(5):1129–1140.
- Schefold JC, Wollersheim T, Grunow JJ, Luedi MM, Z’Graggen WJ, Weber-Carstens S, et al. Muscular weakness and muscle wasting in the critically ill. J Cachexia Sarcopenia Muscle. 2020;11(6):1399–1412.
- González A, Abrigo J, Achiardi O, Simon F, Cabello-Verrugio C. Intensive care unit-acquired weakness and the COVID-19 pandemic: A clinical review. Eur J Transl Myol. 2022;32(3):10511.
- Berger D, Bloechlinger S, von Haehling S, Doehner W, Takala J, Z’Graggen WJ, et al. Dysfunction of respiratory muscles in critically ill patients on the intensive care unit. J Cachexia Sarcopenia Muscle. 2016;7(4):403–412.
- Sanders KM, Kito Y, Hwang SJ, Ward SM. Regulation of gastrointestinal smooth muscle function by interstitial cells. Physiology (Bethesda). 2016;31(5):316–326.
- Hill LT. Gut dysfunction in the critically ill-mechanisms and clinical implications. S Afr J Crit Care. 2013;29(1):11–15.
- Gramlich L, Kichian K, Pinilla J, Rodych NJ, Dhaliwal R, Heyland DK, et al. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients? A systematic review of the literature. Nutrition. 2004;20(10):843–848.
- Hill LT, Hill B, Miller M, Michell WL. The effect of intra-abdominal hypertension on gastro-intestinal function. South African J Crit Care. 2011;27(1):12–19.
- Weijs PJM, Looijaard WGPM, Dekker IM, Stapel SN, Girbes AR, Oudemans-van Straaten HM, et al. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit Care. 2014;18(2):R12.
- Barkhudaryan A, Scherbakov N, Springer J, Doehner W. Cardiac muscle wasting in individuals with cancer cachexia. ESC Heart Fail. 2017;4(4):458–467.
- Springer J, Tschirner A, Haghikia A, von Haehling S, Lal H, Grzesiak A, et al. Prevention of liver cancer cachexia-induced cardiac wasting and heart failure. Eur Heart J. 2014;35(14):932–941.
- Patejdl R, Klawitter F, Walter U, Zanaty K, Schwandner F, Sellmann T, et al. A novel ex vivo model for critical illness neuromyopathy using freshly resected human colon smooth muscle. Sci Rep. 2021;11(1):24249.
- Zhou T, Yang K, Thapa S, Liu H, Wang B, Yu S, et al. Differences in symptom burden among cancer patients with different stages of cachexia. J Pain Symptom Manage. 2017;53(5):919–926.
- Hejazi RA, Zhang Da, McCallum RW. Gastroparesis, pseudoachalasia and impaired intestinal motility as paraneoplastic manifestations of small cell lung cancer. Am J Med Sci. 2009;338(1):69–71.
- Kelly D, Moran C, Maher M, O’Mahony S. Malignancy-associated gastroparesis: an important and overlooked cause of chronic nausea and vomiting. BMJ Case Rep. 2014;2014:bcr2013201815.
- Vaes RDW, Welbers TTJ, Van Dijk DPJ, Rennspiess D, Hausen AZ, Damink SWMO, et al. Intestinal smooth muscle aberrations in pancreatic cancer patients with sarcopenia. JCSM Rapid Commun. 2021;4(2):187–196.
- Formenti P, Umbrello M, Castagna V, Cenci S, Bichi F, Pozzi T, et al. Respiratory and peripheral muscular ultrasound characteristics in ICU COVID 19 ARDS patients. J Crit Care. 2022;67:14–20.
- Wang L, Yang H, Lv G, Fu X, Cheng Y , Zhong X, et al. Association of gastric antrum echodensity and acute gastrointestinal injury in critically ill patients. Nutrients. 2022;14(3):566.
- Dent E, Morley JE, Cruz-Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148–1161.
- Bauer J, Morley JE, Schols AMWJ, Ferrucci L, Cruz-Jentoft AJ, Dent E, et al. Sarcopenia: a time for action. An scwd position paper. J cachexia sarcopenia muscle. 2019;10(5):956–961.
- Gonçalves TJM, Horie LM, Gonçalves SEAB. Diretriz braspen de terapia nutricional no envelhecimento (braspen guideline on nutrition therapy in aging). BRASPEN J. 2019;34(3):2–58.
- Vliet SV, Beals JW, Martinez IG, Skinner SK, Burd NA. Achieving optimal post-exercise muscle protein remodeling in physically active adults through whole food consumption. Nutrients. 2018;10(2):224.
- Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, Helms E, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52(6):376–384.
- Toledo DO, Gonçalves RC, Castro MG. Meta proteica versus disfunção renal na Unidade de Terapia Intensiva. BRASPEN J. 2016;31(4):367–370.
- McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Society of critical care medicine; american society for parenteral and enteral nutrition. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40(2):159–211.
- Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79.
- Fetterplace K, Deane AM, Tierney A, Beach LJ, Knight LD, Presneill J, et al. Targeted full energy and protein delivery in critically ill patients: a pilot randomized controlled trial (FEED Trial). JPEN J Parenter Enteral Nutr. 2018;42(8):1252–1262.
- Pennings B, Boirie Y, Senden JMG, Gijsen AP, Kuipers H, van Loon LJV, et al. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J ClinNutr. 2011;93(5):997–1005.
- Goelen N, Janssen P, Ripken D, van Horssen P, Byloos K, Ghysels S, et al. Effect of protein composition of enteral formula on gastric content volume during continuous feeding: A randomized controlled cross-over study in healthy adults. Clin Nutr. 2021;40(5):2663–2672.
- Hogan S, Steffens D, Rangan A, Solomon M, Carey S. The effect of diets delivered into the gastrointestinal tract on gut motility after colorectal surgery-a systematic review and meta-analysis of randomised controlled trials. Eur J Clin Nutr. 2019;73(10):1331–1342. Eur J Clin Nutr. 2019;3(6):65–68.
- Fonseca LM, Luz MMP, Lacerda A, Correia M, Silva RG. A simplified rehabilitation program for patients undergoing elective colonic surgery--randomized controlled clinical trial. Int J Colorectal Dis. 2011;26(5):609–616.
- Yagmurdur H, Leblebici F. Enteral nutrition preference in critical care: fibre-enriched or fibre-free? Asia Pac J Clin Nutr. 2016;25(4):740–746.
- O’Keefe SJ. The need to reassess dietary fiber requirements in healthy and critically ill patients. Gastroenterol Clin N Am. 2018;47(1):219–229.
- Arkwright JW, Blenman NG, Underhill ID, Maunder SA, Spencer NJ, Costa M, et al. Measurement of muscular activity associated with peristalsis in the human gut using fiber bragg grating arrays. IEEE Sens J. 2012;12(5):113–117.
Keywords
Sarcopenia; Gastrointestinal tract; Protein; Fiber
Cite this article
Toledo DO, Castro MG, de Pinho IC, Prim C, Waitzberg DL. Can sarcopenia also affect the gastrointestinal tract of the critically Ill patient? Clin Stud Gastroenterol Hepatol. 2022;1(1):1–6.
Copyright
© 2022 Diogo Oliveira Toledo. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).


