Abstract
A 69-year-old female with a history of previous STEMI presented to the emergency department with chest pain and EKG changes consistent with anterior STEMI. Left heart catheterization revealed multivessel CAD. She was referred for a CABG. Transthoracic Echocardiography (TTE) with contrast raised concerns for apical pseudoaneurysm. Cardiac Computed Tomography (CT) confirmed the presence of a multi-lobular dissecting pseudoaneurysm and extensive pericardial thickening. This was confirmed at surgery, and she underwent partial pericardiectomy. Repair of the defects was deemed too high risk and adhesion prevented the insertion of any grafts. She subsequently underwent percutaneous revascularization.
Case Presentation
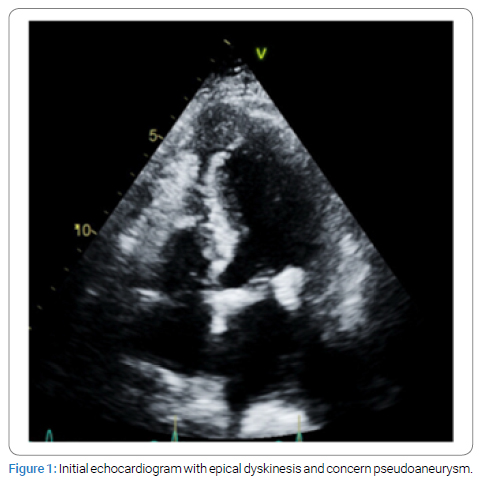
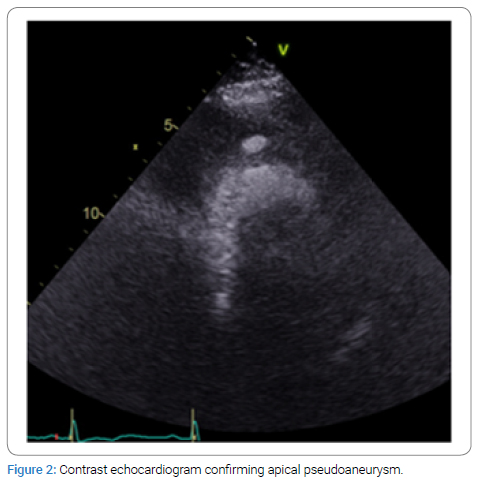
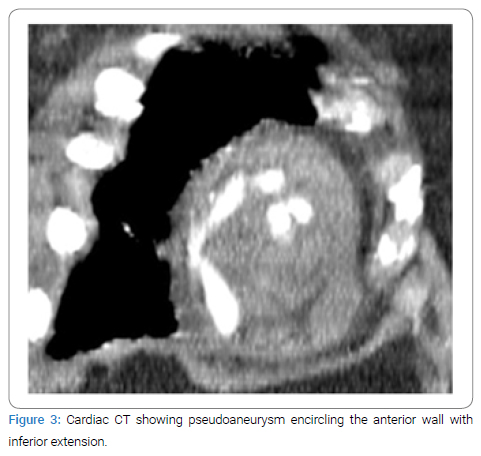
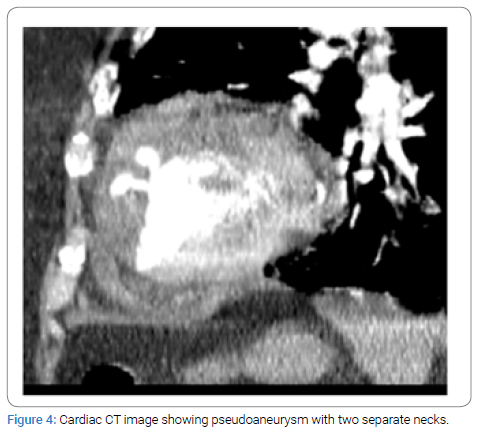
A 69-year-old female with history of previous STEMI presented to the emergency department with chest pain and was found to have EKG changes consistent with ST elevation myocardial infarction. Patient accepted Nitroglycerine and Aspirin in the emergency department. Emergent coronary angiogram revealed multi-vessel coronary artery disease involving both the LAD and LCX. Previous stent in the RCA was visualized but no new stents were placed. Patient was started on Integrilin, admitted to the ICU, and referred to cardiothoracic surgery in the morning. A transthoracic echocardiogram was performed before surgery and found that the apex and apical septum were akinetic to dyskinetic and raised concerns for apical pseudoaneurysm (Figure 1). Radiology read this finding as a LV thrombus, but the cardiology and cardiothoracic surgery team disagreed and ordered an echo with contrast to investigate these findings. The contrast echo confirmed a multilocular apical pseudoaneurysm (Figure 2), but further imaging was still indicated for surgical planning. The patient was unable to tolerate cardiac magnetic resonance imaging so cardiac Computed Tomography (CT) was performed as part of surgical planning. The cardiac CT confirmed the presence of a large pseudoaneurysm encircling the anterior wall with inferior extension and two separate necks (Figure 3 and 4). The patient was taken for surgery 5 days after STEMI presentation for coronary artery bypass grafting and pseudoaneurysm repair.
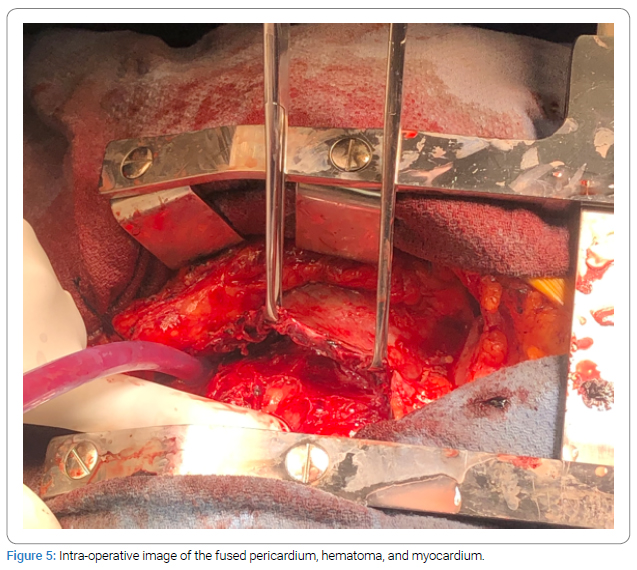
Opening of the pericardium found blood clots encased around the heart. The pericardium was fused in parts to the myocardium below. Preoperative images were reviewed again in this context which in retrospect revealed that this was not actually a LV pseudoaneurysm. The more likely mechanistic process alluded to a chronic Left Ventricular (LV) aneurysm that had ruptured with resultant intramuscular hematoma.
Cardiac imaging specialists were brought into the operating room to discuss the intraoperative findings. As the entire muscle, intramuscular hematoma, and pericardium were fused, the insertion of any patch repairs was not feasible and surgical repair was deemed too high risk. The CABG was similarly discontinued as it would have been impossible to identify a 1 mm to 2 mm coronary artery within the fragmented heart muscle and old hematoma. After a second surgical opinion, the surgery was aborted as to prevent any more harm to the patient.
Patient was brought back to the ICU and extubated 6 hours after the surgery. The cardiology team medically managed the patient’s coronary artery disease. Patient was seen by a physical therapist who recommended a skilled nursing facility, and she was discharged on postoperative day #6. Patient was seen 1 month later by her primary care provider, who noted no significant complaints or physical exam findings. Patient had not followed up with outpatient cardiology at this time. One month later the cardiology team was informed by a family member that the patient had passed away due to covid-pneumonia.
Discussion
Rupture of the left ventricular wall is a rare event in roughly 4% of all Myocardial Infarction (MI) patients that may present with nonspecific symptoms [1–4]. Diagnosing LV pseudoaneurysms has typically been done via transthoracic or transesophageal echocardiography [1,4]. In our case, the presence of LV contrast outside the LV and a narrow-necked connection suggested LV pseudoaneurysm, but the images were difficult to confirm. Cardiac CT helped provide a clearer delineation of the pathology with extensive myocardial dissection and pericardial thickening. Further retro-analysis of the preoperative imaging and dyskinesis of the LV wall was also likely underestimated as it was bounded and thus supported by the pericardium, sternum, and diaphragm. These boundaries provided multiple lobes as a conduit for flow in and out of the initially thought pseudoaneurysm. A similarly contained aneurysm was reported by Si et al. [3], in that the rupture did not extend to the pericardium to invoke cardiac tamponade and had an otherwise normal cardiac function via sonography. This highlights the importance of incorporating other studies such as cardiac MRI and CT with higher sensitivity to not only identify the lesion but also create 3-dimensional models to possibly aid in the surgical repair. In addition, the use of a multidisciplinary team approach with input from cardiac imaging, interventional cardiology, and cardiothoracic surgery will help assess the imaging findings and anticipate surgical challenges in a complicated case such as this.
Surgical repair is the preferred treatment option for LV pseudoaneurysms as this may assist in allowing for revascularization via CABG, restoring ventricular composition and shape, and decreasing any possible volume overload [4,5]. The initial indication for the surgery was correct based on the information and imaging studies available at that time. However, during the operation, it was discovered that the narrowed neck finding in the past studies was more likely an old, chronic, and organized clot that remained as a part of the ventricle wall. Although the culprit vessel was not stented percutaneously on admission, this delay was likely not involved in the formation of the aforementioned finding as these clots were likely from her previous STEMI. The decision to abort further repair of the defect was made with the help of intra-operation transesophageal echocardiography. Therefore, our case is unique in that the presumed new pseudoaneurysm was an old aneurysm that ruptured and was contained by the pericardium, a novel finding that must be considered in future presentations. Any surgical operations would most likely have caused more harm. With this type of lesion, it was deemed best to medically manage the patient rather than further disrupting the contained hematoma. Similar cases previously reported of dissecting intramyocardial hematoma masquerading as LV pseudoaneurysm have demonstrated difficulty of diagnosis relying solely on imaging (Figure 5). These reports and have shown a similar patient course of recovery from initial hemodynamic instability due to spontaneous clotting of rupture site [6–8]. In future cases with similar presentations, we hope that one may look at our images as a reference to exclude a patient from undergoing an invasive surgery when the extent is beyond repair.
Limitations
Patient was not able to tolerate cardiac MRI secondary to excessive movement, which could have possibly helped elucidate some of the pathology in this case. In addition, we were not able to get information regarding the patient’s prognosis and surgery outcome since the patient did not follow-up with cardiology and unfortunately passed away.
Perspective
This case report is written from the perspective of the inpatient cardiology care team at the respective hospital. The research methodology involves observations and interviews.
Conclusion
LV pseudoaneurysm is a known complication of myocardial infarction, especially in cases of delayed treatment. An extensive intramural course is rare and poses a surgical challenge. These challenges can be discussed via an interdisciplinary team approach that was helpful in this complex medical decision-making. Multimodality imaging including both an echocardiogram and CT was required to understand the full extent of the lesion that may assist in reconstructing a 3D model.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
Keywords
LV pseudoaneurysm; Transesophageal echocardiography; Cardiac CT; Interdisciplinary management
Cite this article
Mehdizadeh C, Amatya P, Partow-Navid R, Rao RM, Rothstein D, Ali MW, et al. Left ventricular dissecting pseudoaneurysm with extensive intramural course: diagnostic challenges. J Heart Disord. 2023;2(1):1–4.
Copyright
© 2023 Mehdizadeh C. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).





