Vaccination as Negative and Suspicious Positive Risk of Subacute Sclerosing Panencephalitis (SSPE)
* Takasu Toshiaki;
Miki K;
-
* Takasu Toshiaki: Department of Neurology, Nagaoka-Nishi Hospital, Niigata, Japan.
-
Miki K: Department of Neurology, Nagaoka-Nishi Hospital, Niigata, Japan.
-
Jul 23, 2024 |
-
Volume: 2 |
-
Issue: 1 |
-
Views: 1966 |
-
Downloads: 1201 |
Abstract
This review and meta-analysis ascertained the negative risk of SSPE by vaccination and confirmed the method to further clarify the risk of SSPE. Purpose of present review was to recognize what questions about vaccination been answered as risk of SSPE and what study needed for further clarification. Patients with histories of having had vaccination and not having had measles illness were collected from the literature. Such patient had existed in a total number of 94 in SSPE. Such histories provided by two principal reports were found un-distinguished by odds ratio. The negative risk of SSPE retained by such histories was ascertained with 2 x 2 contingency tables utilizing the reported figure. Phenomena such as the much less ratio of SSPE yearly occurrence per million distributed vaccines than per million estimated measles illnesses; the declined incidence of SSPE from earlier to later years; the increase in proportion of such SSPE patient from 1980 to 1989; the shorter latencies of SSPE from vaccination than from measles; and the increased latencies from earlier to later years; were found reported. Explanation of these phenomena of vaccination remained ambiguous by either subclinical measles or vaccination or both leaving room for suspect of positive risk by vaccination. Conclusion: Negative risk of SSPE was ascertained by the reported histories of having had vaccination and not having had measles illness in case-control studies. Suspicious positive risk of SSPE by such histories was found retained in cases. Case-control study by a multi-varietal analysis with specified variable e.g, by date or by age or else is needed for future clarifying the specified risk of SSPE.
Abbreviations
SSPE: Subacute Sclerosing Panencephalitis; M: Measles; V: Vaccination; NoM: No Measles; NoV: No Vaccination; PNG: Papua New Guinea; EHP: Eastern Highlands Province; GBH: Goroka Base Hospital
Terminology
Positive risk signifies risk with odds ratio values above one. Negative risk signifies risk with odds ratio values below one.
Prologue
SSPE is a progressive neurodegenerative disease caused by the persistence of measles infection which commonly seen in children and young adults [1]. The present reviewers had understood that the precise mechanism of its pathogenesis had not been established yet, but accumulated evidence had suggested that in SSPE measles virus particles had been incompletely eliminated by the immune system and persisted in infected cells in the brain, spreading from cell to cell and eventually culminating in the development of the disease. Those SSPE viruses had been characterized by a defective expression of M protein arising from a highly mutated genome, resultantly interfering with the assembly of new viral particles and their budding. Children with, or exposed to human immunodeficiency virus infection, who contract measles, may be at increased risk of SSPE [2]. The pathogenesis for SSPE had remained poorly understood [3]. Many questions concerning the lack of efficient immune control in the CNS had been still open [4]. Increased capacity of Me V to enter cells [5] and spread [5–7] in the brain had been recently discussed by the mutations of wild-type measles virus proteins [5–7]. After reporting a high incidence and an elevated ratio of late measles in SSPE in Karachi, Pakistan [8,9], the present reviewer Takasu T faced the reported higher incidences and the elevated ratio of early measles of SSPE in PNG [10–12].
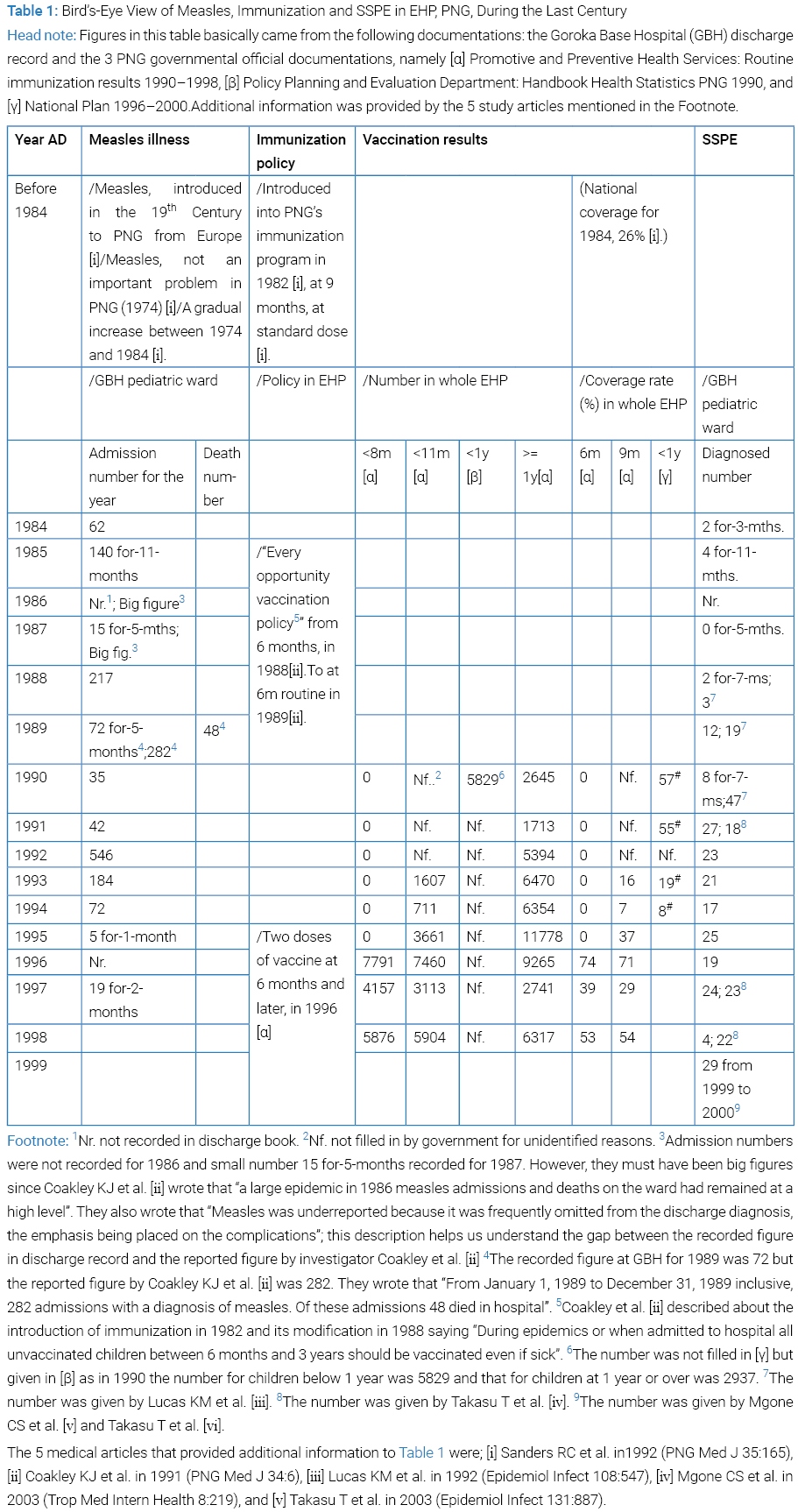
In PNG on the spot in 1997 and 1998 we Takasu T and collaborator Miki K procured the discharge diagnosis record between 1984 and 1998 of measles and SSPE at pediatric ward of GBH, EHP, PNG, and later the PNG governmental data of measles vaccination number and coverage from 1990 to 1998; these records and data were got together, readjusted and summarized in (Table 1). Helped with predecessors’ writings [10–12] and other [13–21] as well as the relevant book chapter (See #in Table 3 Foot note) and also referring to the 3 PNG governmental records ([α], [β] and [γ] in Table 1 Head note) and the 4 WHO’s referred documentations [22–25], we recognized the chronological arriving of measles [15] and SSPE [10–12,18,19,21] to GBH, the introduction of immunization to EHP in 1982 [10,13–16,21–25] and the modification of the policy in 1988 [13,22–24]. Briefly over viewing the table, a large measles epidemic in PNG in 1986 appeared in GBH pediatric ward, where repeated epidemics seen with short time lags. The year 1992 saw the largest admission of measles to the ward. In Goroka at least it was very clear that a predominant feature of the epidemic was the large proportion of measles cases and death occurring in less than 1 year of age [14,15]. Vaccination coverage to infants below 1 year of age showed a drastic decline from 1991 to 1994 in EHP, then recovered and fluctuated until1 1998. The number of SSPE cases at GBH was observed had exhibited a remarkable increase in discharge record in 1989, even during the course of the year [11]. Aiming at investigation, a case-control study work was started with gathering control, its result going to be submitted for publication. A virological study was reported in 2002 [17]. Descriptive clinical and epidemiological data of SSPE patient were reported in 2003 [18,19]. In between, the “probable measles vaccine-associated SSPE” so termed by Okuno et al. [26] as well as the SSPE in which both measles and measles vaccination were positive as histories [27,28] attracted our attention and became targeted in the present review.
Purpose of Review
Ultimate purpose of the reviewer Takasu T’s study is to understand why so high or low incidence of SSPE was observed in some areas or else and what difference in risk was between those areas. Risk encompasses virus, vaccine, host, and environment of host, society and evolution of these. In other words, the ultimate purpose of reviewer is to understand the entire picture of SSPE epidemiologically and in other ways. Purpose of present review was to recognize in what extent questions about vaccination as risk of SSPE had been answered and what study needed for their further clarification.
Material and Method
Reported figures on history of having had vaccination and not having had measles illness in case and control were collected from the literature and analyzed using 2 x 2 contingency table for obtaining Fisher’s exact probability, relative risk and odds ratio. For statistical significance and odds ratio js-STAR_XR +2 x 2 KINSET was utilized.
Background of Programed Immunization In USA, In Japan and in PNG
In North America, measles infection has existed after Europeans introduced it [29]. The first reported outbreaks occurred in 1765 [29]. 1912: Routine reporting of measles cases begins [29]. Measles became a nationally notifiable disease in the US in 1912 [30]. In the 1950s, nearly all children got measles by the time they were fifteen years of age [30]. In 1963, live attenuated [30,31] and inactivated measles vaccines both were licensed [31]. However, the inactivated vaccine was withdrawn because of the atypical measles [31]. In 1968, further attenuated live vaccine (Moratan or Schwarz or Edmonston-Enders strain) began to be distributed [30]. In 1989, a two dose schedule of the MMR vaccine for all children was introduced to the US [32].
In Japan, measles infection had existed for more than one thousand years back for, since 892 AD, measles had been recorded [33]. At least 38 times of outbreaks with intervals between 10 years and 30 years had continued to occur by the end of the Edo Era [33]. Since 1966, both killed and live vaccines had been licensed and generally used [31]. However, the killed vaccine was withdrawn, then the live vaccine replaced by further attenuated live vaccine (Schwarz or Biken-CAM or AIK-C strain) [34]. Since 1978, compulsory regular vaccination had been carried out [31]. In 2006, a two doses schedule of measles vaccination at 1 year and 6 years of age was adopted [33].
The background of immunization against measles in PNG was viewed in (Table 1).
SSPE in USA, in Japan, and in PNG
In 1969, the US national SSPE registry began reporting to gather a total of 375 cases that occurred from 1960 to 1974 [27].
In 1976, the Japan national SSPE registry began reporting to collect a total of 275 cases that occurred from 1966 to 1991[26] [2 or Y in Table 1 Head note].
SSPE diagnosed number at GBH saw a rise during the course of 1989 [11] which continued until at least 1999 [19]. Thus we saw an apparent increase of SSPE forerun by measles epidemics and immunization.
Manning et al. [21] reported in 2011, a series of 22 SSPE cases presenting between November 2007 and July 2009 in Madang Province, PNG, in which the distribution of year of birth of the 22 children with SSPE closely matched the reported annual measles incidence in PNG, including a peak in 2002; writing “Despite relatively stable vaccination coverage between 50% and 65% from 1997 to 2008, there was a substantial increase in the numbers of reported acute measles cases in 2002 with a smaller prior peak in 1999 and 2000”.
Fact about Vaccine
Injection: Measles vaccines are usually injected subcutaneously, but are also effective when injected intramuscularly [25].
Chance of Vaccination: Vaccination had aimed at a time interval of host between after maternal antibody had vanished and before host was exposed to wild-type virus. Vaccination of infants before or at the age of 6 months often fails to induce sero-conversion due to immaturity of the immune system as well as the presence of neutralizing antibodies from mother [25]. Primary vaccination failure occurs in up to 10% to 15% of infants vaccinated at age 9 months [25].
Titer and Dose: Standard or medium or high titer vaccine had been so far attempted. In one study standard titre was defined as 3–4 log10 PFU (Plaque-forming unit), medium titre as 4–5 log10 PFU and high titre as >= 5 log10 PFU [35]. High titre had been defined as > 5.0 log10 PFU or higher in 1989, but changed in 1990 to > 4.7 log10 or higher per human dose [24,35]. The standard volume of measles-containing vaccine first dose (MCV1) is 0.5 ml [25].
Measles immunization before the Age of 9 Months: A number of investigators are completing large studies comparing different strains and potencies of measles vaccines administered to infants prior to the age of 9 months [22]. Preliminary data from these studies were reviewed and discussed by the WHO Research and Development Group and by the WHO Global Advisory Group [22]. They suggest one or more vaccines will be identified which will be suitable of routine use before the age of 9 months in infants at high risk of exposure to measles [22].
Decreased Survival of Increased Titer Vaccine Recipient: In the 1980s, live attenuated measles vaccines of increased titer (> 105 TCID50; namely higher than the virus titer required to infect 50% of host cells in culture) (Wikipedia telling that, as a working estimate, one can assume material with a TCID50 of 1 x 105 TCID50/ml will produce 0.7 x 105 PFUs/ml.) were tested as an approach to overcome the inhibitory effect of maternal antibodies on the infant’s immune response, but it was discontinued after reports of excess mortality in girls who had received these high titer vaccines as compared with girls immunized with standard titer vaccines [25]. Its use was recommended by WHO in 1990 as sufficient data are now available to recommend that “High titre” Edmonston-Zagrev (EZ) measles vaccine be administered at 6 months of age or as soon as possible thereafter in countries where measles before the age of 9 months is a significant cause of death [23]. But it was discontinued after reports of excess mortality in girls who had received these high titre vaccines as compared with girls immunized with standard titer vaccines [25] as it was said that high tire (equal to or greater than 4.7 log10 infectius units per human dose) measles vaccine derived from the original Edmonston measles vaccine isolate should no longer be recommended for use in immunization programmes [24].
Immune Response to Vaccine: Measles vaccine induces both humoral and cellular immune responses similar to those induced by wild type measles virus, although antibody concentrations are usually lower [25].
Host Cell Receptor for Vaccine: The vaccine-strain measles virus enters all nucleated cell via CD46 including neural [36]. Vaccine or laboratory strains have been adapted to grow in common cell lines such as Vero or Hela cells, and were found to use CD46 as a receptor [37]. CD46 was found on the surface of all human cells with the exception of erythrocytes [37]. CD46 protein expressed in, as tissue, cerebral cortex at low score but not in cerebellum or hippocampus or caudate [36]. Mutations in the H protein of measles virus, which occur during adaptation and allow the virus to use CD46 as a receptor, have been identified [37] in contrast to the wild-type virus isolates which cannot use the CD46 receptor [37]. CD46 expressed at relatively low levels by neurons and astrocytes in normal brains [38].
SSPE and CD46: Within brain lesions of SSPE cases, CD46 was either not detected or expressed to a lesser degree by neural cells in contrast, normal levels of CD46 found in SSPE brain tissue distant from the lesion [38]. Using in situ hybridization, mRNAs of both MV (measles virus) nucleocapsid and MV Hemagglutinin (MV-H) were detected in all SSPE lesions, while no or only small amounts of MV-H protein were detected [38]. These findings suggest that the CD46 expression is reduced by the MV infection in lesion of SSPE brain [38].
CD46 RNA: CD46 RNA expressed in, as tissue, cerebral cortex, cerebellum choroid plexus, basal ganglia, thalamus, hypothalamus, midbrain, pons, medulla oblongata, hippocampal formation, spinal cord, white matter, amygdale, and retina in the levels around 30 nTPM (normalized expression of transcripts per kilobase million) [36]. CD46 RNA expressed in, as single cell, neuronal cells (excitatory neuron, inhibitory neuron, cone photoreceptor cells, rod photoreceptor cells, bipolar cells, horizontal cells) at around 50 nTPM levels and glial cells (astrocytes, oligodendrocyte precursor cells, oligodendrocytes, microglial cells, Mueller glial cells) at levels from 20 nTPM to 150 nTPM [36]. Wild-type isolates of measles virus cannot use the CD46 receptor [37].
In Vivo Replication of Vaccine: (The in vivo target cells for vaccine-strain measles virus are not well characterized, but) replication is restricted compared with wild-type virus despite enhanced ability to use widely distributed CD46, as well as SLAM (i.e., CD150), as a receptor [39]. Limited in vivo studies suggest that vaccine-strain and wild-type viruses replicate equally well in the respiratory tract, but that vaccine virus replicates less well in lymphoid tissue resulting in lower levels of virus in circulating PBMCs (viremia), potentially accounting both for less serious disease and less vigorous immune response to infection and in lymphatic cell less vigorously than wild-type virus [39].
Genotype of measles vaccine: Genetic studies had supported that SSPE not to be caused by the vaccine strain [1,17,31,40,41]. All 9 cases of SSPE with history of vaccination gave Genotype D3 in 7 and Genotype D6 in 1 and Genotype E in 1 [40]. Eight clades (A–H) and 23 Genotypes are recognized, based on the sequences of the carboxyl region of the N gene, or the full sequence of the H gene [41]. All measles vaccine strains are Genotype A [41]. There have been no cases of SSPE in whom measles vaccine virus has been isolated [41].
Absence of the tri-residue motif in vaccine: The capacity of wild-type measles virus strain to cause SSPE results from their increased capacity to spread and this is partially due to a tri-residue motif, P64, E84 and A209 (PEA) in their M proteins [6] or P63, E84 and T209 (PET) [6], which is absent in vaccine and laboratory-adapted strains [1,6]. The equivalent residues for vaccine strains are either S64, K89 and T209 (SKT) as in Moratan or PKT [1,6].
Existence of Patient with History of Vaccination
Clinico-epidemiological [19,26–28,42,43] or clinico-epidemio-pathological [40] studies revealed the existence of SSPE patient with histories of vaccination with or without histories of measles illness (Table 2 and Table 3).
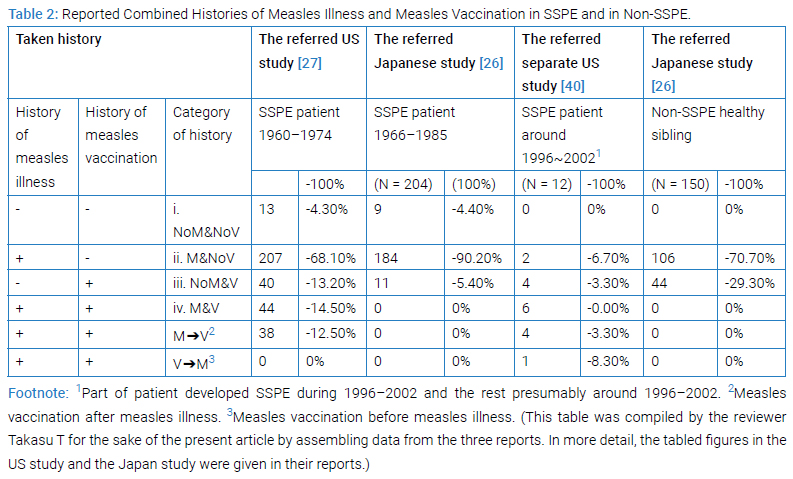
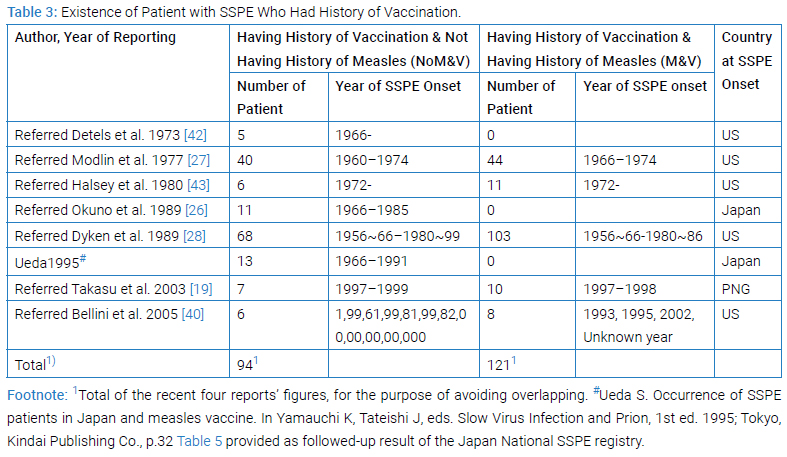
Comprehensibly being viewed, combination of the reported histories of measles illness and measles vaccination gave four categories, i (NoM&NoV), ii (M&NoV), iii (NoM&V) and iv (M&V) in both US [27] and Japan [26] studies (Table 2). Specifically viewing, the proportions of first 3 categories in the US and the Japan studies (actually 4.3% vs. 4.4%; 68.1% vs. 90.2%; and 13.2% vs. 5.4%, respectively) were much similar to each other while those of Category-iv (14.5% vs. 0%) having been decisively different between the two. The sole feasible interpretation of it must be, as the Japanese authors in 1989 mentioned in discussion [26], that all Japanese mothers had been expected to keep a maternity record of health conditions and immunizations for their children and mothers themselves [26], so that all children having history of measles illness must not had received measles vaccine any more, thereby any history having fallen not to Category-iv but to Category-ii. The maternity record system had existed since 1947 [44,45] in Japan and since 1966 the record has been named Maternal and Child Health Handbook [44,45]. In other view, those patients who had been vaccinated could be grouped to two namely Category-iii and Category-iv in the US study subjects while those patients grouped to only one namely Category-iii in Japan study. The separate US study [40] during 1996–2002 gave a pattern of proportion which differed much from that during 1960–1974 by the US registry [27] reflecting the elevated vaccination coverage in later years.
If we focus on the SSPE patients in these studies, as it appeared in (Table 3), the existence of patient of SSPE who had history of vaccination not having history of measles, namely Category-iii history (NoM&V), was evident amounting to 94 in number avoiding overlapping, that was recorded in four reports (Table 3) [28] (#in Table 3 Footnote) [19,40]. These 94 patients had the history of vaccination un-associated with history of measles illness (NoM&V) must have retained potentially positive risk of SSPE. As well, existence of patient of SSPE with history of vaccination having history of measles, namely having Category-iv history (M&V), 121 in number avoiding overlapping, was recorded in three reports (Table 3) [19,28,42]. The 121 patients had vaccination associated with measles illness (M&V) must have retained potentially positive risk of SSPE. In either iii or iv history, so far as potentially risk remains, suspicion remains. Why these patients existed? If the risk be zero, these patients do not exist. Halsey et al. [43], had left a remark that their study could not confirm or rule out the possibility that live measles vaccine might lead to SSPE on rare occasions.
Negative Odds Ratio by History of Vaccination Un-associated with History of Measles Illness
The two reported case-control studies from US [43] and from Japan [26] examined by McNemar (matched pair) test or by χ2 test, respectively, had given a proven evidence for more frequent occurrence of ‘naïve’ namely un-specified measles vaccination (Table 4) that in the 17 out of the 52 cases than from the 63 out of the 96 hospital or playmate controls (p < 0.05 from hospital control and 0.01 from playmate control) or in the 11 out of the 204 cases than from the 44 out of the150 controls (p < 0.01 from healthy controls), respectively; whereby, however, no odds ratio was given in either study.
The present reviewer Takasu T won success in giving ‘naïve’ vaccination (V) as well as un-associated vaccination with measles illness (NoM&V) a proven risk of SSPE together with the values of odds ratio which was obtained by 2 x 2 contingency tables, utilizing the figures given in the two previous reports (Table 4).
The ‘naïve’ vaccination was endowed with the odds ratio of 0.25 or 0.14 in the US and the Japanese studies with Fisher’s exact probability of 0.0001 or 0.0000, respectively (Table 4). This result ascertained the negative risk of SSPE by naïve vaccination. The Category-iii history (NoM&V) in the US study was endowed with Fisher’s exact probability of 0.0000 or 0.0000, and odds ratio of 0.16 or 0.14 in the US and the Japanese studies, respectively (Table 4). This result revealed the negative risk of SSPE by vaccination un-associated with history of measles illness, by which the negative risk was lower than that by ‘naïve’ vaccination.
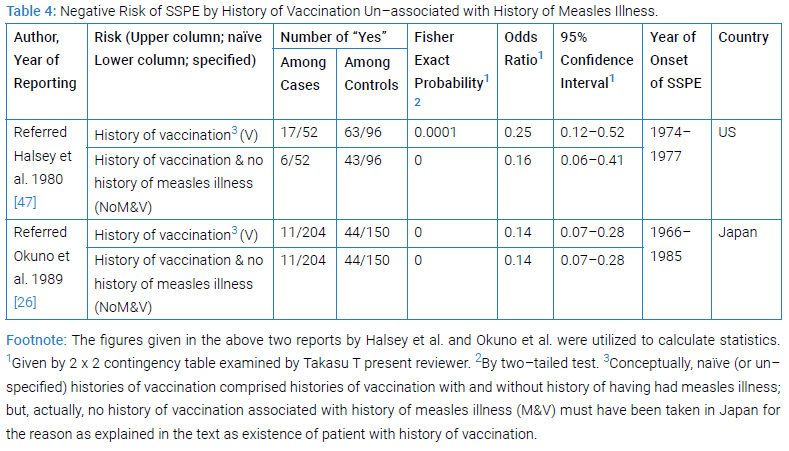
Comments on these achievements: (1) Capability of case-controlling was distinguished: The two studies both had been case-controlled. (2) The odds ratio as almost perfect approximation to relative risk was obtained: In general, relative risk is a best index of causal effect. In rare disease, odds ratio values were good approximates to the risk, the degree of approximation depending on the grade of risk in control (p0). In SSPE, the value of risk among unexposed (i.e., p0) had been as small as < 10–6 since that value among exposed (namely p1) having been 10–6, the highest ever reported [19], so that odds ratio value is nearly equal to relative risk in population. (3) Lower negative risk obtained by specified than by naïve variable: Specified vaccination provided definitely lower negative risk than naïve (i.e., un-specified). (4) Retained risk of SSPE in vaccination: The odds ration values were actually 0.25 for naïve and 0.16 for specified history of vaccination in the US study, meaning the risk of SSPE both in naïve or specified history of vaccination having not been nil though less than in unvaccinated by 75% or 84%; in other words, 25% or 16% of risk retained. (5) Obscure independency of other risk (s): Both analyses for the US and for the Japanese studies were mono-varietal leaving its independency of other risk(s) obscure.
Phenomena of Vaccination in SSPE
Ecological studies focused on Category-iii history (NoM&V) [19,26–28] revealed several phenomena of vaccination in SSPE.
Much less Ratio of SSPE Phenomenon per Vaccine than per Measles Illness: The two reports from the national SSPE registry, the US [26] and the Japan [26], provided with the number of SSPE together with the number of distributed vaccine as representing the best available approximation of the number of vaccine recipients [27]. For the purpose of comparison to this number, the number of SSPE together with the estimated number of measles illness had been provided by both reports.
Ratio of SSPE per Vaccine: The US national SSPE registry gave data enough for us to conceive that, between 1963 and 1974, a total of 36 patients with SSPE with such a history received vaccine and a total of 74 million doses of vaccine were distributed [27]. A ratio of SSPE per vaccine was 0.49 SSPE per million doses [27]. The Japan national SSPE registry gave data enough for us conceive that, between 1971 and 1983, a total of 8 patients with SSPE with such a history received vaccine and a total of 12.84 million dose of vaccine were distributed [26]. The ratio of SSPE per vaccine was 0.62 SSPE per million doses [26].
Ratio of SSPE per Measles Illness: A total of 182 US patients with SSPE with “no history of vaccination (presumed from the original text by the present reviewer) but with a history of measles” were reported between 1960 and 1972 and a total of 29.16 million estimated measles between the same times were reported [27]. The ratio of SSPE per measles was 6.24 SSPE per million measles [27]. A total of 159 Japanese patients with SSPE with no history of vaccination but with a history of measles (M&NoV) and a total of 20.87 million estimated measles cases were reportedbetween1960 and 1981 [26]. The ratio of SSPE per measles was 7.62 SSPE per million measles [26].
Declined Incidence of SSPE Phenomenon After Use of Further Attenuated Live Vaccine Spread: By the US registry followed-up study report [28], “since the early 1970s” the annual incidence (defined as the number of SSPE that occurred in the year and registered later) “declined rapidly” [28]. Namely, “the incidence from 1976–1986 (10.3 patients per year) was much reduced in comparison to the number from 1967-1975 (41.3 patients per year)” [28]. “The number of yearly cases following vaccination has been stable since 1974, whereas the incidence of patient with a measles history has declined steadily”. The decline in the US registry began in 1973–1975; 5 years to 7 years after further attenuated live vaccine began to be distributed in 1968. The Japan registry followed-up study on the registered cases for the 26 years 1966–1991gave a total of 189 SSPE cases from 1976 to 1985, ranging 13–27 cases/year (mean 18.6, SD5.2) and another total of 33 SSPE from 1986 to 1991, ranging 3–8 cases/year (mean 5.5 cases/year)(#Table 3 Footnote). The definite decline of SSPE in the Japan registry appeared began abruptly in 1986”; 8years after compulsory regular immunization with FAL vaccine began in 1978. (#Table 3 Footnote)
Increased Proportion From 1980 to 1989 Phenomenon of SSPE Patient with History of Vaccination: In the followed-up US series emphasized was an increase in the proportion of cases following measles vaccination [28]. The number of yearly cases following vaccination has been stable since 1974, whereas the incidence of patients with a measles history has declined steadily [28]. The relatively larger decrease in the 2 measles groups has led to an increases proportion of patients with SSPE following vaccination. Of the 347 patients with known measles and/or vaccine histories who contracted SSPE prior to 1976, only 46 (13.3% = 46/347 x 100) had received the measles vaccine and had no history of measles. The percentage of such patients after 1975 (24.5% = 22/89 x 100) was significantly higher, (p < 0.01) [28] corresponding to the rapid decline in SSPE incidence from 1967–1975 (41.3 patients per year) to 1976–1986 (10.3 patients per year), and to 1982–1986 (4.2 patients per year) [28]. In the followed-up Japan study no increasing proportion of patient with history of measles was recognized between 1966–1985 and 1966–1991. In the Japanese series the Category-iv history namely the histories of vaccination and measles was nil because vaccination to the host with history of measles had been avoided. The maternal and child health handbook utilized by all mothers and child since 1947 [44,45] enabled the avoidance.
Shorter Latency of SSPE Phenomenon from Vaccination than from Measles Illness: The interval from vaccination or from measles illness to SSPE onset meant the latency of SSPE from vaccination or from measles illness.
The US national registry forerunning had given [27] the mean value (3.3 years) of the latency from vaccination which was lower than the mean value (7.1 years) of the latency from measles (Table 5). The followed-up US national SSPE registry gave data [28] enough for us to be convinced of the latency from vaccination with a history of vaccination but no history of measles (i.e., Vaccine Only)was shorter (p < 0.05) than the latency from measles in the two measles groups Measles Only (Table 5) or Measles and Vaccine.
The Japan national SSPE registry had given data [26] enough for us to nearly be convinced the same as above, namely the latency of SSPE from vaccination was shorter than the latency from measles only (Table 5).The followed-up Japan national SSPE registry gave more precise data (Shown in #of Table 3 Foot note) whereby the conclusion remain dun changed (Table 5). The above situation could have been realized only when measles illness occurred in earlier age and measles vaccination received in later years.
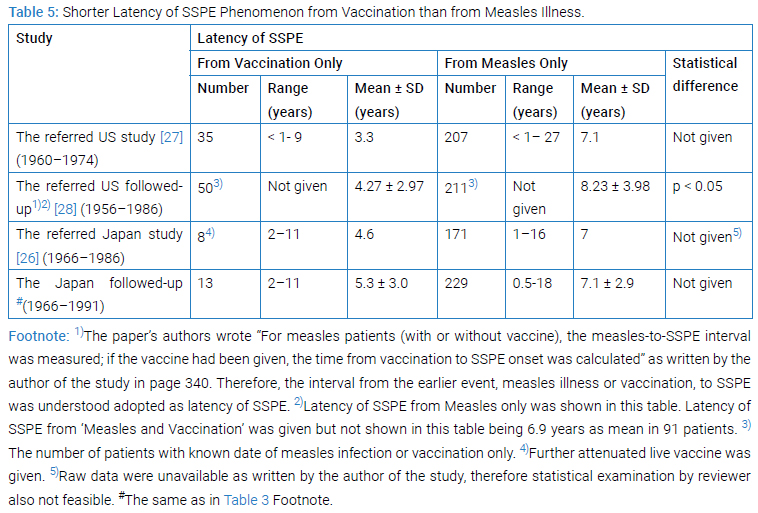
By the way, in the SSPE registered at GBH, EHP, PNG, the latency of SSPE from vaccination among 20 patients ranging between 2.7 years and 14.3 years was 7.0 ± 2.9 as mean ± SD (median 6.0), while the latency of SSPE from measles only among 18 patients ranging between 2.5 years and 11.1 years was 5.9 ± 2.1 as mean ± SD (median 5.6) [19]. This result in GBH was different from those in the US or in Japan. External situations surrounding host at GBH or USA or Japan and/or internal situations of neural cell occupied by persisting mutated measles virus must had made themselves different. In general, latency of SSPE depends on the ages at measles, vaccination and/or SSPE onset, wherein the age at measles had been influenced possibly by natural or social environmental circumstances of host whereas the age at vaccination been determined by artificial choosing or political decision.
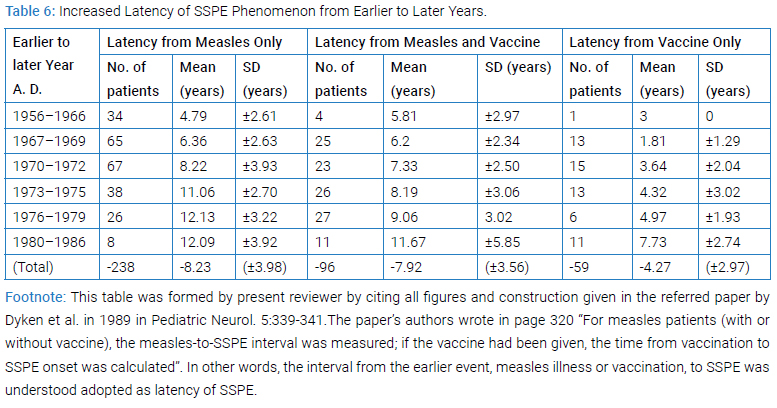
Increased Latency of SSPE Phenomenon from Earlier to Later Years: The follow-up data of the US national registry showed that SSPE latencies had increased (Table 6) from the earlier to later years in all three categories of history of vaccination and/or measles and the increases were statistically significant [28]. This appeared meaning that the processes toward SSPE from vaccination or from measles illness were both controlled by common environmental conditions that can influence upon host, rather than by self-limiting intracellular viral replication alone.
Hypothetical Pathogenesis of SSPE
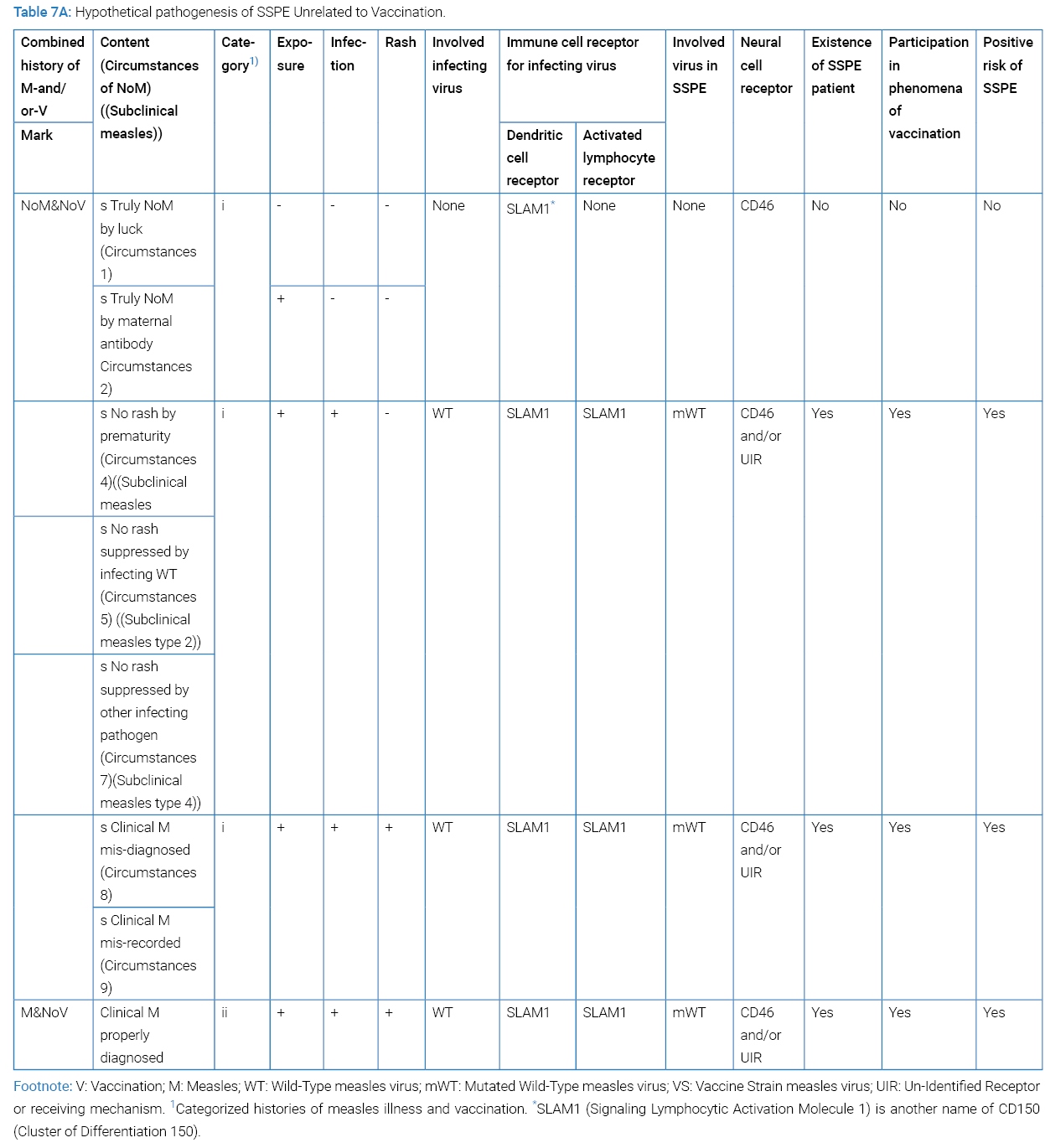
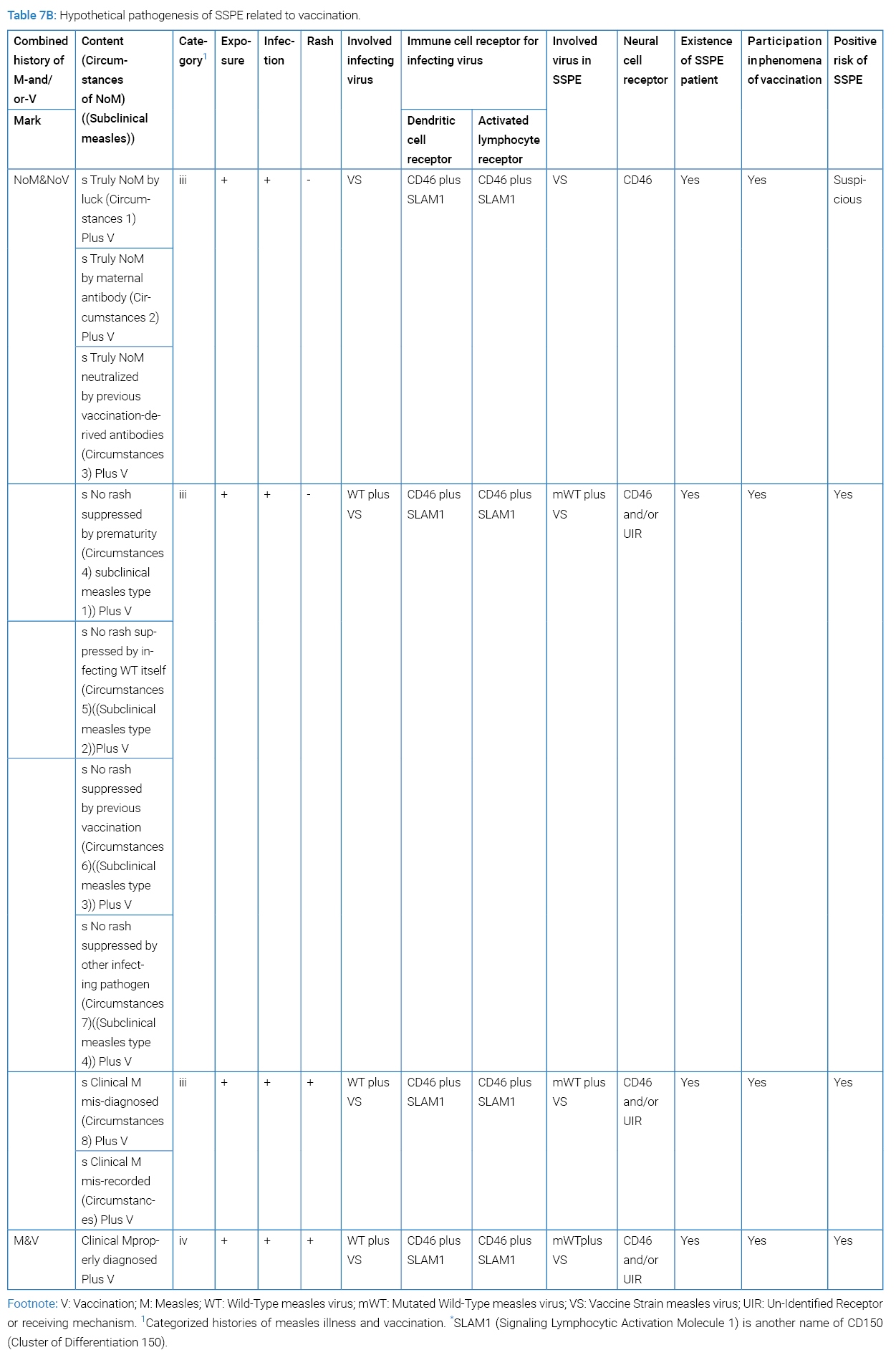
To have SSPE established, mutated measles virus must have entered the brain cell via CD46 or any other unknown receptor or receiving mechanism. SSPE patients have born any one of the four categories of clinical history: where NoM signifying no discerned clinical measles; more directly no discernible measles rash in history. Subclinical measles falls to NoMan divided to Category-I or Category-iii history. Past subclinical infection of host with measles virus could be disclosed by temporally increased anti-measles antibodies [46] or later development of SSPE [47] or theoretically of MIBE (measles inclusion body encephalitis). By the way, a report indicated that asymptomatic measles infection is common but would rarely become a source of transmission because of negative PCR in NPS (nasopharyngeal swab) [48].
Situation behind SSPE was divided by having had measles illness or not having had measles illness (Table 7A). The latter was divided by nine circumstances of “NoM” and further by having received vaccination or not having received vaccination (Table 7B). To each circumstance, positive risk of SSPE was tried evaluated. Circumstances 4, 5, 6 and 7 could be called subclinical measles type 1, type 2, type 3 and type 4. The reviewer’s study of participation by each circumstance in the phenomena of vaccination had not been accomplished yet. Epidemiology of subclinical measles is a difficult task since its content being heterogeneous. Epidemiological evidence by subclinical measles stays within the limit of possibility. More specification of vaccination, for example by date or by age or else, seems necessary for ascertaining hidden positive risk of SSPE by vaccine.
Future Analysis
Future analysis should be case-controlled, be with specified variables, and be multi-varietal.
- It should be case-controlled. The three such studies [26,42,43] alone were capable of giving statistically significant risk. In general the royal road next to intervention is cohort study or case-control study (Nakamura Y. A Text book of Epidemiology without Tears. 4th edn. Tokyo: Igaku-Shoin Ltd. 2020.).
- It should be done with specified variables. The ‘naïve’ measles illness was specified by age in the first case-control study on the risk of SSPE [42], namely as the measles 1 year or under, that led success in proving its significance: the ’naïve’ (I.e., un-specified) positive history of measles was not significant but the history of measles at 1 year or under was significant, i.e., p < 0.01 with χ2 test [42].
- It should be multi-varietal since incidence of SSPE differed so widely in different regions that region-dependent multiple factors expected confounding. To detect independent risks of each other, multi-varietal analysis is needed. For example, Kondo K reported in J Clin Exp Med. 1988;146:799 from relevance to SSPE, that the patient with histories of the four risks (i.e., measles under 1 year with OR of 7.31, repeated infection with OR of 4.00, head injury with OR of 2.79, and convulsion with OR of 2.90) was revealed exposed to a huge risk with OR of 273.6 when all four thrown into an equation.
Conclusion of Meta-Analytic Review
The negative relative risk and odds ratios demonstrated by the history of vaccination without history of having had measles illness proved vaccination as negative risk of SSPE. In addition,
- Existence of the patients with SSPE with history of vaccination without history of measles retains vaccination as a suspicious positive risk of SSPE. Likewise, the much less ratio of SSPE patients from vaccine than from measles, the declined incidence of SSPE after further attenuated live vaccines spread, the increase in proportion from 1980 to 1989 of patients with SSPE with history of vaccination, and the shorter latency of SSPE from vaccination than from measles retains vaccination; retain vaccination as another suspicious positive risk of SSPE.
- Studies in future should be case-controlled, be performed with specified variable by date or by age or by combination or by sequence, and be conducted by multi-varietal analysis in order to identify specified variable as risk of SSPE.
Conflict of Interest
The present reviewer proclaims that what was written in the article is in no conflict of interest against the cited author or against the collaborator.
Acknowledgment
The author thanks the PNG Department of Health staffs for helping the study work by the Japanese team. The author thanks the PNGIMR and GBH staffs for allowing the study-work and providing information. In particular, the author thanks Joyce M Mgone, then chief pediatrician at GBH, for her suggestion and collaboration with the Japanese team in investigating ward record; and Charles S Mgone, then deputy-director of PNGIMR and head of its molecular genetics division. The author thanks Toshiki Nishimura for his help in literature survey during 1995.The author expresses thanks to the editorial manager of this journal for her patient gentle reminding.
References
- Jafri SK, Kumar R, Ibrahim SH. Subacute sclerosing panencephalitis - current perspectives. Pediatric Health Med Ther. 2018;9:67–71.
- Mekki M, Eley G, Hardie D, Wilmshurst JM. Subacute sclerosing panencephalitis: Clinical phenotype, epidemiology, and preventive interventions. Develop Med Child Neurol. 2019;61(10):1139–1144.
- Griffin DE. Measles virus and the nervous system. Handb Clin Neurol. 2014:123:577–590.
- Reuter D, Schneider-Schaulies J. Measles virus infection of the CNS: human disease, animal models, and approaches to therapy. Med Microbiol Immunol. 2010;199(3):261–271.
- Angius F, Smuts H, Rybkina K, Stelitano D, Eley B, Wilmshurst J, et al. Analysis of a subacute sclerosing panencephalitis genotype B3 virus from the 2009–2010 South African measles epidemic shows that hyperfusogenic F proteins contribute to measles virus infection in the brain. J Virol. 2019;93(4):e01700–e01718.
- Kweder H, Ainouze M, Brunel J, Gerlier D, Manet E, Bukland R. Measles virus: identification in the M protein primary sequence of a potential molecular marker for subacute sclerosing panencephalitis. Adv Virol. 2015;2015:769837.
- Watanabe S, Shirogane Y, Sato Y, Hashiguchi T, Yanagi Y. New insights into measles virus brain infections. Trend Microbiol. 2019;27(2):164–175.
- Kondo K, Takasu T, Akhtar Ahmed. Neurological diseases in Karachi, Pakistan--elevated occurrence of subacute sclerosing panencephalitis. Neuroepidemiology. 1988;7(2):66–80.
- Takasu T, Kondo K, Ahmed A, Yoshikawa Y, Yamanouchi K, Tsuchiya M, et al. Elevated ratio of late measles among subacute sclerosingpanencephalitis patients in Karachi, Pakistan. Neuroepidemiology. 1992;11(4–6):282–287.
- Sanders RC, Brian M, Rongap A, Watt PD, Alpers MP. High incidence of subacute sclerosing panencephalitis (SSPE) in young children in Papua New Guinea. Med J Australia. 1990;153(11–12):740.
- Lucas KM, Sanders RC, Rongap A, Rongap T, Pinai S, Alpers MP. Subacute sclerosing panencephalitis (SSPE) in Papua New Guinea: a high incidence in young children. Epidemiol Infect. 1992;108(3):547–553.
- Lucas K. Subacute sclerosing panencephalitis in Papua New Guinea. PNG Med J.1993;36(3):185–186.
- Coakley KJ, Coakley CA, Spooner V, Smith TA, Javati A, Kajoi M. A review of measles admissions and deaths in the paediatric ward of Goroka Base Hospital during 1989. PNG MedJ.1991;34(1):6–12.
- Rogers S, Sanders RC, Alpers MP. Immunogenicity of standard dose Edmonston-Zagreb measles vaccine in highland Papua New Guinean children from four months of age. J Trop Med Hyg. 1991;94(2):88–91.
- Sanders R. The changing pattern of measles in Papua New Guinea - past and future. PNG Med J. 1992;35(3):165–168.
- Bass AG. Vaccines in the national immunization programme. PNG MedJ.1993;36(2);141–157.
- Miki K, Komase K, Mgone CS, Kawanishi R, Iijima M, Mgone JM, et al. Molecular analysis of measles virus genome derived from SSPE and acute measles patients in Papua, New Guinea. J Med Virol. 2002;68(1):105–112.
- Mgone CS, Mgone JM, Takasu T, Miki K, Kawanishi R, Asuo PG, et al. Clinical presentation of subacute sclerosing panencephalitis in Papua New Guinea. Trop Med Intern Health. 2003;8(3):219–227.
- Takasu T, Mgone JM, Mgone CS, Miki K, Komase K, Namae H, et al. A continuing high incidence of subacute sclerosing panencephalitis (SSPE) in the Eastern Highlands of Papua New Guinea. Epidem Infect. 2003;131(2):887–898.
- Takasu. (Japanese) Neuroinfectious disease and international cooperation – SSPE in Karachi and Goroka (Papua New Guinea). Neuroinfection. 2009;14:13–24.
- Manning L, Laman M, Edoni H, Mueller I, Karunajeewa HA, Smith D, et al. Subacute sclerosing panencephalitis in Papua New Guinean children: The cost of continuing inadequate measles vaccine coverage. PLoS Neglected Tropical Diseases. 2011;5(1):e932.
- WHO.EPI: Global Advisory Group. Measles immunization before the age of 9 months. WklyEpidem Rec. 1989;64:5–10.
- WHO. EPI: Global Advisory Group. Part I. Measles control; measles immunization before the age of 9 months. WklyEpidem Rec. 1990;65:5–11.
- WHO.EPI. Safety of high-titre measles vaccines. WklyEpidem Rec. 1992;67(48):357–361.
- WHO. Measles vaccines: WHO position paper – April 2017.WklyEpidem Rec. 2017;92(17):205–228.
- Okuno Y, Nakao T, Ishida N, Konno T, Mizutani H, Fukuyama Y, et al. Incidence of subacute sclerosing panencephalitis following measles and measles vaccination in Japan. Intern J Epidemol. 1989;18(3):684–689.
- Modlin JF, Jabbour JT, Witte JJ, Halsey NA. Epidemiologic studies of measles, measles vaccine, and subacute sclerosing panencephalitis. Pediatrics. 1977;59(4):505–512.
- Dyken PR, Cunningham SC, Ward LC. Changing character of subacute sclerosing panencephalitis in the United States. Pediatr Neurol. 1989;5(6):339–341.
- Online Public Health. The history of measles in the United States. Measles in the U. S. an ongoing battle. 2019.
- CDC. Measles History.2020.
- Nakayama T. (Japanese) Feature articles 2. Present and future of vaccine. 3. Measles vaccine. Uirusu.2009;59:257–266.
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS. Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR-04);1–34.
- Komase K. (Japanese) Current status of measles in Japan and progress towards measles elimination. Modern Medicine.2015; 61: 81–90.
- Shishido R. (Japanese) 5. Measles vaccine. National Institute of Preventive Hygiene Scholars Society Students’ Association, editors. In: Japanese Vaccines, revised 2nd edn. Tokyo and Kyoto: Maruzen Publishing Co. 1977;87–103.
- Knudsen KM, Aaby P, Whittle H, Rowe M, Samb B, Simondon F, et al. Child mortality following standard, medium or high titre measles immunization in West Africa. Intern J Epidemiol. 1996;25(3):665–673.
- Thul PJ, Lindskog C, The human protein atlas: A spatial map of the human proteome. 2018;27(1):233–244.
- Lin L-T, Richardson CD. The host cell receptors for measles virus and their interaction with the viral hemagglutinin (H) protein. Viruses. 2016;8(9):250.
- Ogata A, Czub S, Ogata S, Cosby SL, McQuaid S, Budka H, et al. Absence of measles virus receptor (CD46) in lesions of subacute sclerosing panencephalitis brains. Acta Neuropathol. 1997;94(5):444–449.
- Griffin DE. Measles vaccine. Viral Immunol. 2018;31(2):86–95.
- Bellini WJ, Rota JS, Lowe LE, Katz RS, Dyken PR, Zaki SR, et al. Subacute sclerosing panencephalitis: more cases of this fatal disease are prevented by measles immunization than was previously recognized. J Infect Dis. 2005;192(10):1686–1693.
- Campbell H, Andrew N, Brown KE, Miller E. Review of the effect of measles vaccination on the epidemiology of SSPE. Intern J Epidem. 2007;36(6):1334–1348.
- Detels R, Brody JA, McNew J, Edgar AH. Further epidemiological studies of subacute sclerosing panencephalitis. Lancet. 1973;2(7819):11–14.
- Halsey NA, Modlin JF, Jabbour JT, Dubey L, Eddins D, Ludwig DD. Risk factors in subacute sclerosing panencephalitis: A case-control study. Am J Epidem. 1980;111(4):415–424.
- Nakamura Y. Maternal and Child Health Handbook in Japan. JMAJ 2020;53:259–265.
- Takeuchi J, Sakagami Y, Perez RC. The mother and child health handbook in Japan as a health promotion tool: An overview of its history, contents, use, benefits, and Global influence. Glob Pediatr Health. 2016;3:2333794X16649884.
- Pedersen IR, Mordhorst CH, Glikmann G, von Magnus H. Subclinical measles infection in vaccinated seropositive individuals in arctic Greenland. Vaccine. 1989;7(4):345–348.
- Wendorf KA, Winter K, Zipprich J, Schechter R, Hacker JK, Preas C, et al. Subacute sclerosing panencephalitis: the devastating measles complication that might be more common than previously estimated. Clin Infect Dis. 2017;65(2):226–232.
- Sonoda S, Nakayama T. Detection of measles virus genome in lymphocytes from asymptomatic healthy children. J Med Virol. 2001;65(2):381–387.
Keywords
Subacute sclerosing panencephalitis; Vaccination; Subclinical measles; Epidemiology
Cite this article
Takasu T, Miki K. Vaccination as negative and suspicious positive risk of subacute sclerosing panencephalitis (SSPE). 2024;2(1):1–13.
Copyright
© 2024 Takasu Toshiaki. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).