Evaluation of 2,3-Disubstituted Quinazolones for their Antimicrobial Study by the Standardized Procedures as Recommended by the National Committee for Clinical Laboratory Standards (NCCLS)
* Pandey VK;
* Soniya Singh;
* Ritu Awasthi;
-
* Pandey VK: Department of Chemistry, University of Lucknow, Lucknow, India.
-
* Soniya Singh: Department of Chemistry, University of Lucknow, Lucknow, India.
-
* Ritu Awasthi: Department of Chemistry, University of Lucknow, Lucknow, India.
-
Feb 03, 2023 |
-
Volume: 1 |
-
Issue: 1 |
-
Views: 809 |
-
Downloads: 813 |
Abstract
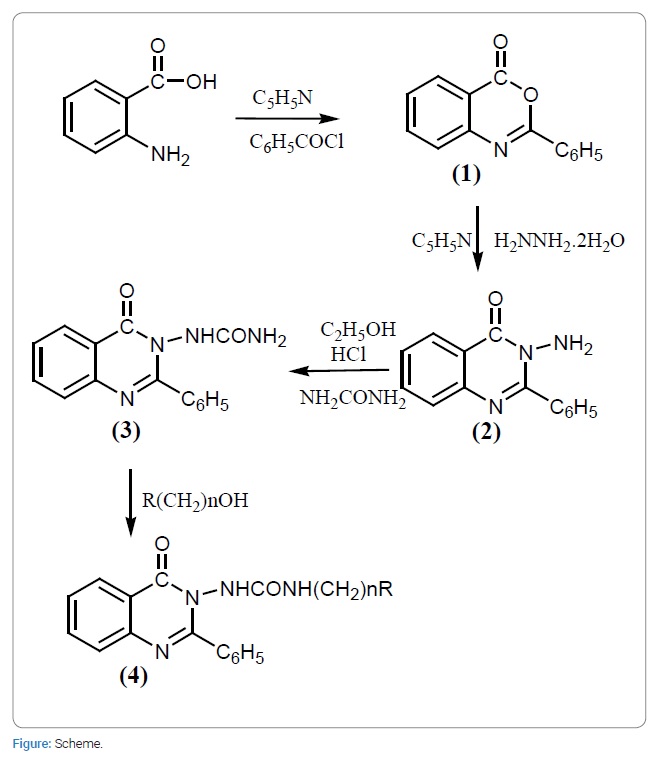
Heating under reflux a mixture of anthranilic acid and benzoyl chloride in dry pyridine afforded 2-phenyl-4H-benzo [d] [1,3] oxazine-4-one(1). Reaction of (1) with an excess equivalent of hydrazine hydrate in dry pyridine solvent furnished 3-amino-2-phenylquinazolin-4(3H)-one(2) which on reaction with carbamide in ethanol containing catalytic quantity of HCl yielded 1-(4-oxo-2-phenylquinazolin-3(4H)-one)(3). Interaction of (3) with arylamido/imidoalcanolsin conc. H2SO4 generated targets compounds(4) designated as N- {[3-(4-oxo-2-phenylquinazolin-3(4H)-yl)ureido] alkyl}arylamides/imides and were evaluated for their antimicrobial activity against four bacterial and four fungal strains.
Introduction
Literature survey reveals that the quinazole compounds have been extensively investigated in several health areas [1–8]. Interest in quinazoles has increased manifolds because of their association with anticancer activity. The antitumour properties of some such compounds have been reported in culture of L1210 cells [4]. Recently, several research papers describing the antiviral and anti-microbial activities of quinazoles have appeared. Thus, the quinazolyl benzimidazoles were found antivirally active against Japanese Encephalitis virus (JEV), a highly pathogenic virus afflicting mostly children with high mortality rate and Herpes simplex virus (HSV-I). Low to moderate order of antiviral activity was observed against both the animal viruses. Quinazole derivatives containing isoquinoline and thiadiazole nuclei were found to exhibit antiviral and antifungal properties.
These compounds were synthesized for their antifungal activity against Fusarium solanis a casual organism of Guava Wilt Disease (GWD). One quinazole compound of this category was found to show percent inhibition of fungal zone to the extent of 70% while others showed low order of antifungal activity [9]. In addition, thiadiaquinazolones were found active against JEV and HSV-I in vitro [10]. Thus, it is evident that the quinazole compounds find greater applications in several health areas. The significant diverse pharmological properties exhibited by quinazole compounds led the authors to undertake the synthesis of N-{[3-(4-oxo-2-phenylquinazolin-3(4H)-yl) ureido]alkyl} arylamides/imides for their antimicrobial activity (4) involving four bacterial and four fungal strains by the standardized methods as recommended by the National Committee for Clinical Laboratory Standards (NCCLS) (Figure).
Experimental Section
1-(4-oxo-2-phenylquinazolin-3(4H)-urea) (3)
3-Amino-2phenylquinazoline-4-(3H)-one (2) (0.05 mole) and urea (carbamide) (0.05 mole) in absolute ethanol containing catalytic amount of concentrated hydrochloric acid (2 ml) were heated under reflux for two hours in such a manner that the reaction mixture was free from atmospheric moisture. Subsequently, solvent was removed by distillation and after washing with cold water, was dried at 100oC. It was recrystallized from acetone as light brown crystalline mass. The analytically pure sample melted at 180oC to 181oC, yield 81%.
N- {[3-(4-oxo-2-phenylquinazolin-3(4H)-yl) ureido] alkyl} arylamides/imides (4)
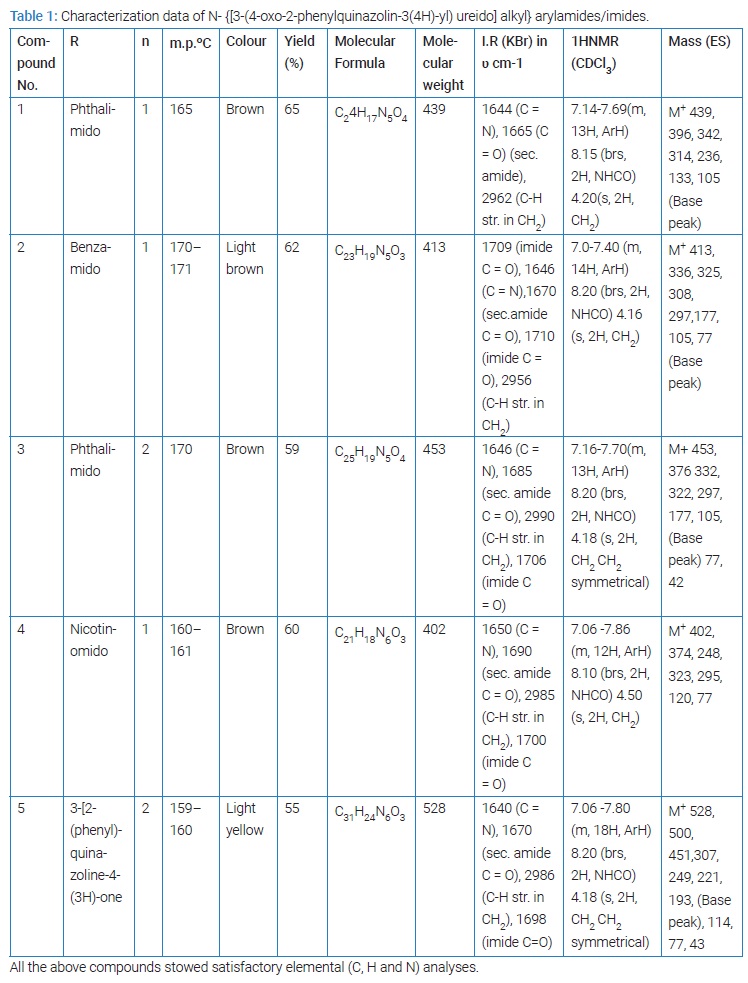
1-(4-Oxo-2-phenylquinazolin-3(4H)-urea) (3) (0.02 mole) and an electrophilic reagent; N-(hydroxy alkyl compound) viz; N-(hydroxy methyl) phthalimide/ N-(hydroxy methyl)- benzamide/N-(hydroxy methyl)-nicotinamide/N-(hydroxy ethyl) phthalimide/ 2-phenylquinazolin-4-(3H)-one (0.02 mole)were finely powdered by grinding together and dissolved in concentrated sulphuric acid by stirring vigorously and carefully [11]. While dissolving, the contents were occasionally cooled so that the reaction temperature did not exceed 10oC. A dark solution resulted which was subjected for further stirring mechanically. The acidic solution thus obtained was refrigerated overnight and subsequently poured in ice cold water (~250 ml) slowly with constant stirring. Precipitated solid mass was allowed to settle down and separated by filtration. The solid thus obtained, was washed successively with cold water in order to remove any sulphonated product and dried in vacuo overnight. It was recrystallized from diluted ethanol using animal charcoal as the decolorizing agent. The characterization data of the compound thus synthesized are recorded in (Table 1).
Biological Activity
Antimicrobial activity was performed as per the literature method [12–14].
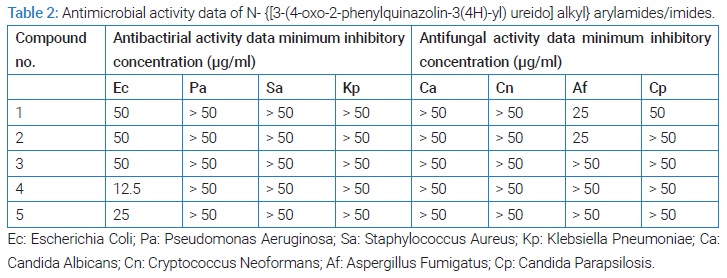
The antimicrobial activity data of the investigational compounds are recorded in (Table 2).
Materials and Methods
For antibacterial activity, testing was done in peptone broth and for antifungal activity, the experimental compounds were tested employing the tube dilution technique. A 1.0 mg/ml solution of the tested compound was obtained by the dissolution in dimethyl sulphoxide solvent.
The Minimum Inhibitory Concentration (MIC) of the investigational compound after incubating at 28oC was recorded after 72/96 hours post incubation.
Results and Discussions
The antimicrobial activity data reported in (Table 2) clearly indicate that all the five quinozolone compounds displayed some measurable degree of antibacterial activity against only one strain of bacteriai.e., against Escherichia coli (Ec). These quinozolone derivatives could not provoke any measurable level of antibacterial activity against other bacterial strains. Quinozolone compounds have been comparatively less extensively investigated in this health area, however, in recent past there are some scattered reports of quinozolones as antibacterial agents.
These compounds were found not to exhibit any inhibitory effect as is evident from the antifungal activity data recorded in (Table 2). Five substituents were made in the quinozolone nucleus along with common substituent (C6H5-) at position -2. As such these compounds do not warrant further investigation as far as antifungal activity is concerned. In order to explore the further possibility. Two attempts seem to be worthwhile. First, other pharmacophoric groups should be introduced at position 2 and 3 of the quinozolone nucleus and second, such compounds should be assayed against other fungal strains.
Conclusion
Amido/imidoalkyl groups have been introduced into the basic molecular architecture with the anticipation that the introduction of such groups might enhance the polarity character of new compounds thus facilitating their dissolution in water and other polar solvents making bioevaluation more feasible. Antibiotics belonging to the penicillin and cephalosporin classes are being uninterruptedly used in fighting the bacterial diseases since a longtime. However, emergence of resistance has become a problematic one and this emergence has increased worldwide during the past few years giving rise to an urgent need for new and more effective chemical candidate molecules. Since some of our compounds are showing promising antimicrobial activity selectivity and a majority of antibiotics and azoles group of antifungal in the arsenal of a physician are developing resistance, therefore in order to meet this challenge new agents are urgently required. These compounds need further investigations involving inclusion of more bacterial and fungal strains.
Acknowledgment
The authors express their sincere thanks to the Head, Department of Chemistry, Lucknow for providing necessary laboratory facilities and to the Director, Central Drug Research Institute (CDRI) for providing elemental, spectral and biological activity data.
Conflict of Interest
The author declares no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Pandey VK, Mukesh M, Kumar A, Trivedi N. An investigation leading to preparation of tetrahydro-quinazoline derivatives involving ureidoalkylation and α-amidoalkylation reactions. Indian J Chem. 2008;47(12):1910–1914.
- Pandey VK, Yadav AS. Synthesis of 4-oxo-2-phenyl-4H-quinazolin-3-carboxylic acid [(aryl-amido/imido)alkyl]-amides and their antimicrobial activity. Indian J Heterocycl Chem. 2008;17(3):271–272.
- VK Pandey, J Kumar, SK Saxena, R Mukesh, MN Joshi, S Bajpai. Synthesis, Characterization and antiviral activity of 2,3-disubstituted quinazolones. Journal of the Indian Chemical Society. 2008;39(19):593–597.
- Tiwari AK, Mishra AK, Bajpai A, Mishra P, Sharma RK, Pandey VK, et al. Synthesis and pharmacological study of novel pyrido-quinazolone analogues as anti-fungal, antibacterial, and anticancer agents. Bioorg Med Chem Lett. 2006;16(17):4581–4585.
- Bishnoi A, Awasthi R, Pandey VK, Tiwari AK, Awasthi NK, Srivastava K, et al. Drug design based on the principle of conjunction: synthesis, characterization and antihyperglycemic activity study of 3- [2- (3- aroyl- 2, 4- diphenyl- 3 , 4- dihydro- 2h [1, 3] oxazino [5, 6-h] quinolin- 6- yl) ethyl] 2-phenyl- quinazolin- 4(3h)- ones. Int J Drug Discov. 2009;1(2):52–55.
- Pandey VK. Possible antiparkinsonian compounds synthesis of 2-styryl-3-arylthiouryl-3,4-dihydro-4-oxoquinazolines. Current Science. 1986;55(5):243–246.
- Singh N, Pandey VK, Gupta R. Synthesis, characterisation and antihypertensive activity of some 2,7,8-substituted-11H-pyrido [2,1-b] quinazolones. Der Pharma Chemica. 2015;7(6):305–311.
- Pandey VK and Gupta M. Quinazolyl-thiazoles as CNS acting agents Acta Pharm. 1996;46(1):51–59.
- Pandey VK, Pathak L, Mishra SK. Synthesis and characterisation of isoquinolinyl quinazolines and a study of their antiviral and antifungal activities. Ind J Chem. 2005;44(9):1940–1943.
- Pandey VK, Tusi S, Tusi Z, Raghubir R, Dixit M, Joshi MN, et al. Thiadiazolyl quinazolones as potential antiviral and antihypertensive agents. Ind J Chem. 2004;43:180–183.
- Tiwari SS, Pandey VK. Possible antiparkinsonian compounds. VII. Synthesis of amidomethylation products with N-hydroxymethylnicotinamide and their conversion into quaternary ammonium iodides". J Indian Chem Soc. 1975;52(2):166–167.
- Varma RS, Nobles WL. Substituted N-amino methyl isatins. J Med Chem. 1967;10(3):510–513.
- Pandeya SN, Sriram D, Nath G, De Clercq E. Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin derivatives with 3-amino-2-methylmercapto quinazolin-4(3H)-one. Pharm Acta Helv. 1999;74(1):11–17.
- Pandey VK, Upadhyay M, Gupta VD, Tandon M, et al. Benzimidazolyl quinolinyl mercaptotriazoles as potential antimicrobial and antiviral agents. Acta Pharm. 2005;55(1):47–56.
Keywords
Arylamido/imidoalcanols; Seeded broth; Quinazolone compounds; Nutrient agarslants
Cite this article
Pandey VK, Singh S, Awasthi R. Evaluation of 2,3-disubstituted quinazolones for their antimicrobial study by the standardized procedures as recommended by the national committee for clinical laboratory standards (NCCLS). Arch Adv Biomed Res. 2023;1(1):1–4.
Copyright
© 2023 Soniya Singh, Pandey VK, Ritu Awasthi. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).



